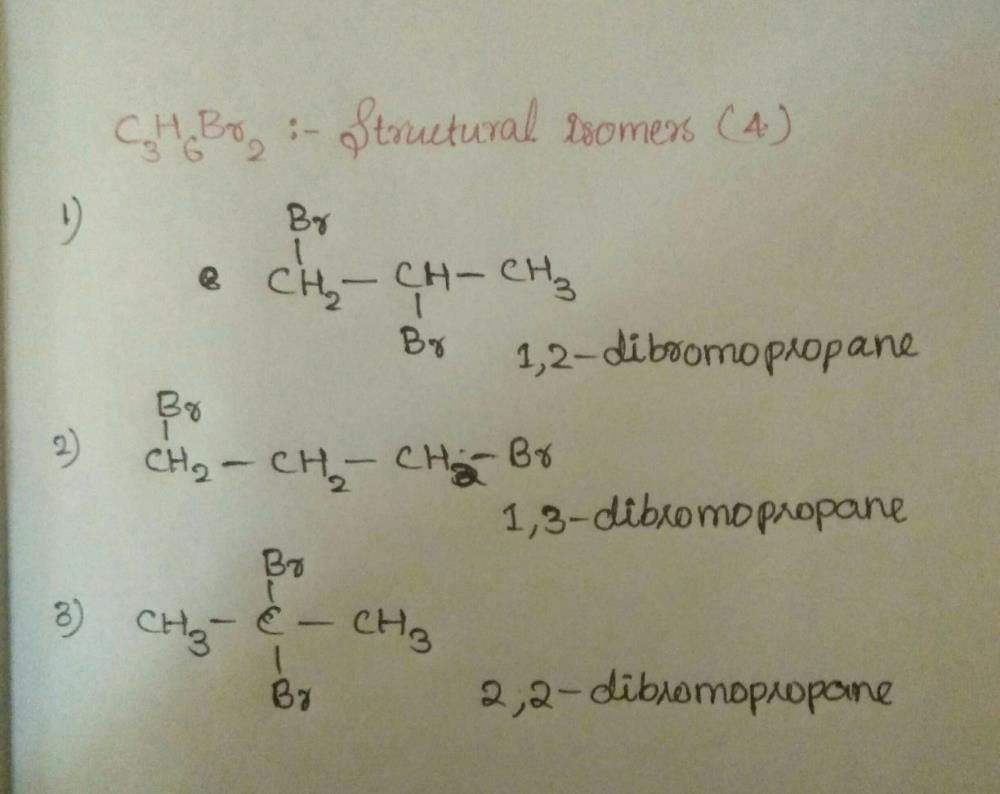Class 11 Exam > Class 11 Questions > C3h6br2 can show?
Start Learning for Free
C3h6br2 can show?
Community Answer
C3h6br2 can show?
Class 11 Chemistry
Introduction to C3H6Br2
C3H6Br2 is the chemical formula for 1,2-dibromopropane. It is an organic compound belonging to the class of haloalkanes. The molecule consists of three carbon atoms, six hydrogen atoms, and two bromine atoms.
Physical Properties of C3H6Br2
- Molecular weight: 201.88 g/mol
- Appearance: Clear, colorless liquid
- Boiling point: 96-99°C
- Melting point: -95°C
- Density: 1.81 g/cm³
Chemical Reactions of C3H6Br2
1. Hydrolysis
- C3H6Br2 can undergo hydrolysis in the presence of a strong base or water to form an alcohol and a bromide ion.
- The reaction is as follows:
C3H6Br2 + H2O → C3H7OH + Br^-
2. Elimination Reaction
- C3H6Br2 can undergo elimination reactions to form an alkene.
- In the presence of a strong base like sodium ethoxide (NaOC2H5) or potassium tert-butoxide (KOtBu), 1,2-dibromopropane can eliminate a hydrogen atom from one carbon and a bromine atom from the adjacent carbon, resulting in the formation of propene.
- The reaction is as follows:
C3H6Br2 + KOtBu → C3H6 + KBr + HBr
3. Nucleophilic Substitution Reactions
- C3H6Br2 can undergo nucleophilic substitution reactions with various nucleophiles.
- In the presence of a nucleophile such as hydroxide ion (OH^-), thiourea (CS(NH2)2), or ammonia (NH3), 1,2-dibromopropane can undergo substitution reactions to form different compounds depending on the nucleophile used.
- For example, with hydroxide ion, the reaction is as follows:
C3H6Br2 + OH^- → C3H5OH + Br^-
Conclusion
C3H6Br2, or 1,2-dibromopropane, is an organic compound that can undergo various chemical reactions such as hydrolysis, elimination, and nucleophilic substitution. These reactions result in the formation of different compounds, including alcohols and alkene. Understanding the reactivity of C3H6Br2 is essential in organic chemistry as it allows for the synthesis of new compounds and the understanding of reaction mechanisms.
Introduction to C3H6Br2
C3H6Br2 is the chemical formula for 1,2-dibromopropane. It is an organic compound belonging to the class of haloalkanes. The molecule consists of three carbon atoms, six hydrogen atoms, and two bromine atoms.
Physical Properties of C3H6Br2
- Molecular weight: 201.88 g/mol
- Appearance: Clear, colorless liquid
- Boiling point: 96-99°C
- Melting point: -95°C
- Density: 1.81 g/cm³
Chemical Reactions of C3H6Br2
1. Hydrolysis
- C3H6Br2 can undergo hydrolysis in the presence of a strong base or water to form an alcohol and a bromide ion.
- The reaction is as follows:
C3H6Br2 + H2O → C3H7OH + Br^-
2. Elimination Reaction
- C3H6Br2 can undergo elimination reactions to form an alkene.
- In the presence of a strong base like sodium ethoxide (NaOC2H5) or potassium tert-butoxide (KOtBu), 1,2-dibromopropane can eliminate a hydrogen atom from one carbon and a bromine atom from the adjacent carbon, resulting in the formation of propene.
- The reaction is as follows:
C3H6Br2 + KOtBu → C3H6 + KBr + HBr
3. Nucleophilic Substitution Reactions
- C3H6Br2 can undergo nucleophilic substitution reactions with various nucleophiles.
- In the presence of a nucleophile such as hydroxide ion (OH^-), thiourea (CS(NH2)2), or ammonia (NH3), 1,2-dibromopropane can undergo substitution reactions to form different compounds depending on the nucleophile used.
- For example, with hydroxide ion, the reaction is as follows:
C3H6Br2 + OH^- → C3H5OH + Br^-
Conclusion
C3H6Br2, or 1,2-dibromopropane, is an organic compound that can undergo various chemical reactions such as hydrolysis, elimination, and nucleophilic substitution. These reactions result in the formation of different compounds, including alcohols and alkene. Understanding the reactivity of C3H6Br2 is essential in organic chemistry as it allows for the synthesis of new compounds and the understanding of reaction mechanisms.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Question Description
C3h6br2 can show? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about C3h6br2 can show? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for C3h6br2 can show?.
C3h6br2 can show? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about C3h6br2 can show? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for C3h6br2 can show?.
Solutions for C3h6br2 can show? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of C3h6br2 can show? defined & explained in the simplest way possible. Besides giving the explanation of
C3h6br2 can show?, a detailed solution for C3h6br2 can show? has been provided alongside types of C3h6br2 can show? theory, EduRev gives you an
ample number of questions to practice C3h6br2 can show? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.