Physics Exam > Physics Questions > T-S diagram for a Carnot’s cycle isSele...
Start Learning for Free
T-S diagram for a Carnot’s cycle is
Select one:
Select one:
- a)quadrilateral types but not rectangle
- b)circle
- c)ellipse
- d)rectangle
Correct answer is option 'D'. Can you explain this answer?
Most Upvoted Answer
T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral ty...
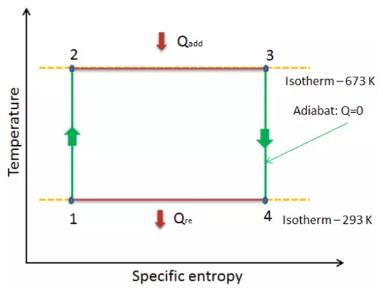
A Carnot cycle can be represented in a T-S diagram, with T along the y-axis and S (entropy) along the x-axis. The isotherms are parallel horizontal lines at Tc and Th. The isentropes are parallel vertical lines (by definition they are of constant entropy). Thus, a Carnot cycle is represented by a rectangle.
Free Test
FREE
| Start Free Test |
Community Answer
T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral ty...
The T-S (temperature-entropy) diagram for a Carnot cycle is a graph that shows the changes in temperature and entropy of a working fluid as it undergoes a Carnot cycle.
In a Carnot cycle, the working fluid is initially at a high temperature and pressure (state 1), then undergoes an isothermal expansion process (state 2), followed by an adiabatic expansion process (state 3), then an isothermal compression process (state 4), and finally an adiabatic compression process back to the initial state (state 1).
The T-S diagram for a Carnot cycle consists of two isothermal lines (horizontal lines) and two adiabatic lines (curved lines). The isothermal lines represent the isothermal processes, where the temperature of the working fluid remains constant. The adiabatic lines represent the adiabatic processes, where there is no heat transfer to or from the working fluid.
The diagram starts at state 1, where the working fluid is at a high temperature and pressure. From state 1 to state 2, the working fluid undergoes an isothermal expansion process, represented by a horizontal line at the high temperature. The entropy of the working fluid increases as it expands.
From state 2 to state 3, the working fluid undergoes an adiabatic expansion process, represented by a curved line. The temperature of the working fluid decreases, and the entropy continues to increase.
From state 3 to state 4, the working fluid undergoes an isothermal compression process, represented by a horizontal line at the low temperature. The entropy of the working fluid decreases as it is compressed.
Finally, from state 4 back to state 1, the working fluid undergoes an adiabatic compression process, represented by a curved line. The temperature of the working fluid increases, and the entropy continues to decrease.
The T-S diagram for a Carnot cycle forms a rectangle shape in the T-S plane, with the two isothermal lines at the top and bottom and the two adiabatic lines on the sides. The area enclosed by the cycle represents the net work done by the working fluid during the cycle.
In a Carnot cycle, the working fluid is initially at a high temperature and pressure (state 1), then undergoes an isothermal expansion process (state 2), followed by an adiabatic expansion process (state 3), then an isothermal compression process (state 4), and finally an adiabatic compression process back to the initial state (state 1).
The T-S diagram for a Carnot cycle consists of two isothermal lines (horizontal lines) and two adiabatic lines (curved lines). The isothermal lines represent the isothermal processes, where the temperature of the working fluid remains constant. The adiabatic lines represent the adiabatic processes, where there is no heat transfer to or from the working fluid.
The diagram starts at state 1, where the working fluid is at a high temperature and pressure. From state 1 to state 2, the working fluid undergoes an isothermal expansion process, represented by a horizontal line at the high temperature. The entropy of the working fluid increases as it expands.
From state 2 to state 3, the working fluid undergoes an adiabatic expansion process, represented by a curved line. The temperature of the working fluid decreases, and the entropy continues to increase.
From state 3 to state 4, the working fluid undergoes an isothermal compression process, represented by a horizontal line at the low temperature. The entropy of the working fluid decreases as it is compressed.
Finally, from state 4 back to state 1, the working fluid undergoes an adiabatic compression process, represented by a curved line. The temperature of the working fluid increases, and the entropy continues to decrease.
The T-S diagram for a Carnot cycle forms a rectangle shape in the T-S plane, with the two isothermal lines at the top and bottom and the two adiabatic lines on the sides. The area enclosed by the cycle represents the net work done by the working fluid during the cycle.

|
Explore Courses for Physics exam
|

|
Similar Physics Doubts
Question Description
T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? for Physics 2025 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Physics 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer?.
T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? for Physics 2025 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Physics 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer?.
Solutions for T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice T-S diagram for a Carnot’s cycle isSelect one:a)quadrilateral types but not rectangleb)circlec)ellipsed)rectangleCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.




















