JEE Exam > JEE Questions > A system of greater disorder of molecules is ...
Start Learning for Free
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.
Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution
Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. ΔTband depression of F.Pt. ΔTf of a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, ΔTb and ΔTf are proportional to the molality of the solute in the solution.
The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, 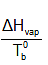 is almost constant .
is almost constant .
[Troutan’s Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ]
For solutes undergoing change of molecular state is solution (ionization or association), the observed ΔT values differ from the calculate ones using the above relations. In such situations, the relationships are modified as 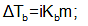
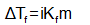
Where i = Van’t –Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.
Q.
To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solution
- a)decreases to a constant value
- b)increases to a constant value
- c)increases first and then decreases
- d)remains constant because HgI2 is sparingly soluble in water.
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
A system of greater disorder of molecules is more probable. The disord...
The number of mole particle decreases from 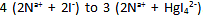
Hence, the Colligative property will decrease or, the vapour pressure will increase to a constant value until NaI is completely consumed.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer?
Question Description
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer?.
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer?.
Solutions for A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer?, a detailed solution for A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vaporizes to form a more disordered gas. When a solulte is present , there is additional contribution to the entropy of the liquid due to increase randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas.Thus , a solute (non volatile) lowers the vapour pressure of a liquid, and hence a higher booing point of the solution Similarly, the greater randomness of the solution opposes the tendency to freeze. In consequence, a lower the temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution . Elevation of B.Pt. Tband depression of F.Pt. Tfof a solution are the colligative properties which depend only on the concentration of particles of the solute, not their identity.For dilute solutions, Tband Tfare proportional to the molality of the solute in the solution.The vaues of Kb and Kf do depend on the properties of the solvent. For liquids, is almost constant . [Troutans Rule , this constant for most of the Unassociated liquids (not having any strong bonding like Hydrogen bonding in the liquid state) is equal to 90J/mol. ] For solutes undergoing change of molecular state is solution (ionization or association), the observed T values differ from the calculate ones using the above relations. In such situations, the relationships are modified as Where i = Vant Hoff factor, greater than unity for ionization and smaller than unity for association of the solute molecules.Q.To aqueous solution of Nal, increasing amounts of solid HgI2 is added. The vapor pressure of the solutiona) decreases to a constant valueb) increases to a constant valuec) increases first and then decreasesd) remains constant because HgI2 is sparingly soluble in water.Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.