JEE Exam > JEE Questions > Passage IA and B are two structural isomers w...
Start Learning for Free
Passage I
A and B are two structural isomers with their molecular formula C7H14O and both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or B with hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2O gives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.
Q.
Which of the following statement regarding A and B is incorrect?
- a)Only A will change the colour of dichromate solution not B
- b)C on reaction with (CH3COO)2Hg/H2O followed by NaBH4 gives B
- c)C on treatment with B2H6/H2O2 followed by alkaline work-up gives A
- d)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same product
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Passage IA and B are two structural isomers with their molecular formu...
Both A and B are alcohols with one degree of unsaturation but none of them decolourise Br2 - H2O solution,hence they are cyclic.
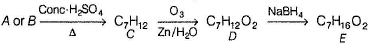
E has only one methyl group and gives iodoform test hence, E may be :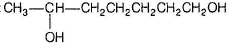
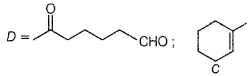
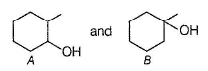
A has a chiral carbon but B does not.
B is a tertiary alcohol, does not react with SOCI2.
E has only one methyl group and gives iodoform test hence, E may be :
A has a chiral carbon but B does not.
B is a tertiary alcohol, does not react with SOCI2.
Most Upvoted Answer
Passage IA and B are two structural isomers with their molecular formu...
Incorrect Statement regarding A and B
Statement B: C on reaction with (CH3COO)2Hg/H2O followed by NaBH4 gives B
Explanation:
- The given statement is incorrect because the reaction described actually leads to the formation of compound D (C7H12O2) from compound C (C7H12), not B.
- The reaction involves the conversion of C to D through the intermediate compound formed by the reaction with (CH3COO)2Hg/H2O followed by NaBH4.
- Hence, the statement B does not correctly represent the reaction pathway of C to form D.
Statement B: C on reaction with (CH3COO)2Hg/H2O followed by NaBH4 gives B
Explanation:
- The given statement is incorrect because the reaction described actually leads to the formation of compound D (C7H12O2) from compound C (C7H12), not B.
- The reaction involves the conversion of C to D through the intermediate compound formed by the reaction with (CH3COO)2Hg/H2O followed by NaBH4.
- Hence, the statement B does not correctly represent the reaction pathway of C to form D.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer?
Question Description
Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer?.
Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is incorrect?a)Only A will change the colour of dichromate solution not Bb)C on reactionwith (CH3COO)2Hg/H2Ofollowed by NaBH4 gives Bc)C on treatment with B2H6/H2O2 followed by alkaline work-up gives Ad)Treating either A or 8 with SOCI2 followed by the reduction with LiAIH4 results in the formation of same productCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























