JEE Exam > JEE Questions > Comprehension Type Direction(Q. Nos. 19-24) T...
Start Learning for Free
Comprehension Type Direction
(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
A and B are two structural isomers with their molecular formula C7H14O and both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or B with hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2O gives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.
Q.
Which of the following statement regarding A and B is correct?
- a)Both A and B are capable of showing optical activity
- b)Only A can show enantiomerism, not B
- c)Only B can show enantiomerism not A
- d)Neither A nor B show enantiomerism
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 pa...
Both A and B are alcohols with one degree of unsaturation but none of them decolourise Br2 - H2O solution,hence they are cyclic.
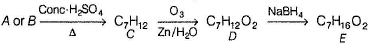
E has only one methyl group and gives iodoform test hence, E may be :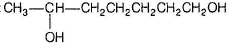
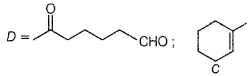
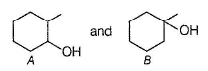
A has a chiral carbon but B does not.
E has only one methyl group and gives iodoform test hence, E may be :
A has a chiral carbon but B does not.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer?
Question Description
Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer?.
Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Comprehension Type Direction(Q. Nos. 19-24) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).Passage IA and B are two structural isomers with their molecular formula C7H14Oand both gives-off effervescence on heating with Na-metal. However, neither A nor B decolourise brown colour of Br2-H2O. Treating either A or Bwith hot conc, sulphuric acid solution results in the form ation of the same compound C (C7H12). C on treatment with O3 followed by Zn-H2Ogives D (C7H12O2). D gives E (C7H16O2) on treatm ent with NaBH4. E has only one methyl group and forms yellow ppt with I2/KOH. Also, A gives delayed turbidity on treatment with conc. HCI/ZnCI2 while B gives immediate turbidity on similar treatment.Q.Which of the following statement regarding A and B is correct?a)Both A and B are capable of showing optical activityb)Only A can show enantiomerism, not Bc)Only B can show enantiomerism not Ad)Neither A nor B show enantiomerismCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























