Chemistry Exam > Chemistry Questions > Which of the following statements most accura...
Start Learning for Free
Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?
- a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixture
- b)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical properties
- c)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvable
- d)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexane
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which of the following statements most accurately describes the stereo...
In the below given diagram of trans-1,3-dichlorohexane the chlorines cannot be positioned diaxial or diequatorial but can only be axial and equatorial or vice versa. Additionally, if they were diastereomers or structural isomers, then they could be separated by physical means.
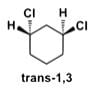
Most Upvoted Answer
Which of the following statements most accurately describes the stereo...
Explanation:
Stereochemistry refers to the three-dimensional arrangement of atoms in a molecule and how it affects the physical and chemical properties of the molecule. In the case of cyclohexanes, there are different isomers with different stereochemistries.
Conformational Isomers of Trans-1,2-dichlorocyclohexane:
- Trans-1,2-dichlorocyclohexane has two different conformers, which are not interconvertible but resolvable.
- The two conformers are mirror images of each other and are therefore enantiomers.
- Enantiomers have identical physical and chemical properties except for their interaction with plane-polarized light.
- Therefore, the two conformers will rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixture.
Diaxial and Diequatorial Forms of Trans-1,3-dichlorohexane:
- Trans-1,3-dichlorohexane has two different conformers, which are interconvertible and therefore not resolvable.
- The two conformers have different physical properties due to their different stereochemistry, but they cannot be separated by physical means.
- Therefore, the diaxial and diequatorial forms of trans-1,3-dichlorohexane cannot be separated by their differing physical properties.
Chirality and Symmetry in Cyclohexanes:
- Chirality refers to the property of a molecule that is non-superimposable on its mirror image.
- A molecule with a plane of symmetry is achiral, meaning it is superimposable on its mirror image.
- Only cis-1,4-dichlorocyclohexane has a plane of symmetry and is therefore achiral.
- Cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexane, meaning they are stereoisomers that are not mirror images of each other.
Therefore, option C is the correct answer as it accurately describes the stereochemistry between the various cyclohexanes.
Stereochemistry refers to the three-dimensional arrangement of atoms in a molecule and how it affects the physical and chemical properties of the molecule. In the case of cyclohexanes, there are different isomers with different stereochemistries.
Conformational Isomers of Trans-1,2-dichlorocyclohexane:
- Trans-1,2-dichlorocyclohexane has two different conformers, which are not interconvertible but resolvable.
- The two conformers are mirror images of each other and are therefore enantiomers.
- Enantiomers have identical physical and chemical properties except for their interaction with plane-polarized light.
- Therefore, the two conformers will rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixture.
Diaxial and Diequatorial Forms of Trans-1,3-dichlorohexane:
- Trans-1,3-dichlorohexane has two different conformers, which are interconvertible and therefore not resolvable.
- The two conformers have different physical properties due to their different stereochemistry, but they cannot be separated by physical means.
- Therefore, the diaxial and diequatorial forms of trans-1,3-dichlorohexane cannot be separated by their differing physical properties.
Chirality and Symmetry in Cyclohexanes:
- Chirality refers to the property of a molecule that is non-superimposable on its mirror image.
- A molecule with a plane of symmetry is achiral, meaning it is superimposable on its mirror image.
- Only cis-1,4-dichlorocyclohexane has a plane of symmetry and is therefore achiral.
- Cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexane, meaning they are stereoisomers that are not mirror images of each other.
Therefore, option C is the correct answer as it accurately describes the stereochemistry between the various cyclohexanes.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer?
Question Description
Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer?.
Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following statements most accurately describes the stereochemistry between the various cyclohexanes?a)Cis-1,2-dichlorocyclohexane and trans-1,2-dichlorocyclohexane rotate plane-polarized light in opposite directions, and together in equal proportions form a racemic mixtureb)The diaxial and diequatorial forms of trans-1,3-dichlorohexane can be separated by their differing physical propertiesc)The conformational isomers of trans-1,2-dichlorocyclohexane are enantiomers, which are not interconvertible, but resolvabled)Only cis-1,4-dichlorocyclohexane is achiral due to a plane of symmetry, and cis-1,4-dichlorocyclohexane is diastereomeric to trans-1,4-dichlorocyclohexaneCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















