Chemistry Exam > Chemistry Questions > What is the relation between wave number of I...
Start Learning for Free
What is the relation between wave number of IR absorption and the reduced mass?
- a)Wave number is directly proportional to reduced mass
- b)Wave number is inversely proportional to reduced mass
- c)Wave number is independent of the reduced mass
- d)Wave number is directly proportional to square of reduced mass
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
What is the relation between wave number of IR absorption and the redu...
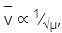 , frequency is directly proportional to wave number. So, wave number is inversely proportional to reduced mass as shown in the above relation.
, frequency is directly proportional to wave number. So, wave number is inversely proportional to reduced mass as shown in the above relation.Free Test
FREE
| Start Free Test |
Community Answer
What is the relation between wave number of IR absorption and the redu...
The relation between wave number of IR absorption and the reduced mass can be understood by considering the basic principles of molecular vibrations and the mathematical expression for the wave number.
1. Understanding Molecular Vibrations:
Infrared (IR) absorption spectroscopy is a technique used to study the vibrational motion of molecules. Molecules can vibrate in different ways, such as stretching, bending, and twisting. These vibrations correspond to different energy levels, and when a molecule absorbs infrared radiation, it undergoes a transition from one vibrational level to another.
2. The Wave Number:
The wave number (ν) is a characteristic parameter used in IR spectroscopy to describe the energy of the absorbed radiation. It is defined as the reciprocal of the wavelength (λ) and is expressed in units of reciprocal centimeters (cm^-1). The wave number is directly proportional to the energy of the absorbed radiation.
3. The Reduced Mass:
The reduced mass (μ) is a concept used in quantum mechanics to describe the effective mass of a system of two or more particles. It takes into account the masses of the individual particles and their relative motion. The reduced mass is inversely proportional to the square root of the total mass of the system.
4. The Relation Between Wave Number and Reduced Mass:
The wave number of an IR absorption is inversely proportional to the reduced mass of the vibrating system. This can be explained by considering the mathematical expression for the wave number:
ν = (1/2πc) * √(k/μ)
Where ν is the wave number, c is the speed of light, k is the force constant of the bond, and μ is the reduced mass.
From this equation, we can see that the wave number is inversely proportional to the square root of the reduced mass. As the reduced mass increases, the square root term decreases, leading to a decrease in the wave number. Conversely, as the reduced mass decreases, the square root term increases, resulting in an increase in the wave number.
Therefore, the correct answer is option B: Wave number is inversely proportional to the reduced mass. This relationship holds true for molecular vibrations in IR spectroscopy.
1. Understanding Molecular Vibrations:
Infrared (IR) absorption spectroscopy is a technique used to study the vibrational motion of molecules. Molecules can vibrate in different ways, such as stretching, bending, and twisting. These vibrations correspond to different energy levels, and when a molecule absorbs infrared radiation, it undergoes a transition from one vibrational level to another.
2. The Wave Number:
The wave number (ν) is a characteristic parameter used in IR spectroscopy to describe the energy of the absorbed radiation. It is defined as the reciprocal of the wavelength (λ) and is expressed in units of reciprocal centimeters (cm^-1). The wave number is directly proportional to the energy of the absorbed radiation.
3. The Reduced Mass:
The reduced mass (μ) is a concept used in quantum mechanics to describe the effective mass of a system of two or more particles. It takes into account the masses of the individual particles and their relative motion. The reduced mass is inversely proportional to the square root of the total mass of the system.
4. The Relation Between Wave Number and Reduced Mass:
The wave number of an IR absorption is inversely proportional to the reduced mass of the vibrating system. This can be explained by considering the mathematical expression for the wave number:
ν = (1/2πc) * √(k/μ)
Where ν is the wave number, c is the speed of light, k is the force constant of the bond, and μ is the reduced mass.
From this equation, we can see that the wave number is inversely proportional to the square root of the reduced mass. As the reduced mass increases, the square root term decreases, leading to a decrease in the wave number. Conversely, as the reduced mass decreases, the square root term increases, resulting in an increase in the wave number.
Therefore, the correct answer is option B: Wave number is inversely proportional to the reduced mass. This relationship holds true for molecular vibrations in IR spectroscopy.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer?
Question Description
What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer?.
What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer?.
Solutions for What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice What is the relation between wave number of IR absorption and the reduced mass?a)Wave number is directly proportional to reduced massb)Wave number is inversely proportional to reduced massc)Wave number is independent of the reduced massd)Wave number is directly proportional to square of reduced massCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















