Chemistry Exam > Chemistry Questions > What do you think is the relationship between...
Start Learning for Free
What do you think is the relationship between bond order and bond length?
- a)Directly proportional
- b)Indirectly proportional
- c)No relation
- d)Cannot predict
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
What do you think is the relationship between bond order and bond leng...
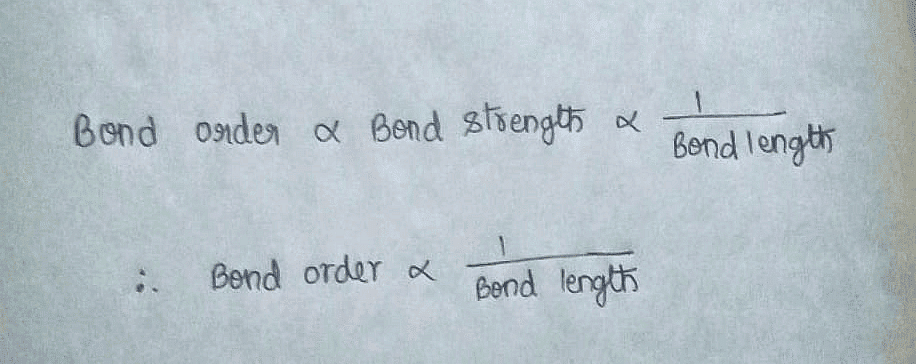
Free Test
FREE
| Start Free Test |
Community Answer
What do you think is the relationship between bond order and bond leng...
The Relationship Between Bond Order and Bond Length
Introduction:
Bond order is a measure of the strength or stability of a chemical bond between two atoms in a molecule. It indicates the number of electron pairs shared between two atoms. On the other hand, bond length refers to the distance between the nuclei of two bonded atoms.
Bond Order:
Bond order is determined by the number of bonding electrons divided by the number of bonding positions available. It provides information about the stability and strength of a bond. A higher bond order indicates a stronger bond and a shorter bond length.
Bond Length:
Bond length is influenced by several factors, including the size of the atoms involved, the number of bonding electrons, and the strength of the bond. A shorter bond length indicates a stronger bond.
The Relationship:
The relationship between bond order and bond length is inversely proportional. This means that as the bond order increases, the bond length decreases, and vice versa.
Explanation:
When two atoms form a bond, they share electrons to achieve a stable electron configuration. The more electron pairs shared between the two atoms, the stronger the bond becomes. As a result, the bond length decreases.
Electron Repulsion:
As more electrons are added to a bond, electrostatic repulsion between the electrons increases. This repulsion forces the bonded atoms closer together to minimize the repulsive forces.
Multiple Bonding:
In molecules with multiple bonds, such as double or triple bonds, the bond order is higher than in single bonds. This is because multiple bonds involve the sharing of more electron pairs. As a result, the bond length is shorter in multiple bonds compared to single bonds.
Example:
Let's consider the case of carbon dioxide (CO2). Each carbon-oxygen bond in CO2 is a double bond, meaning it has a bond order of 2. The carbon-oxygen bond length in CO2 is shorter than the carbon-oxygen bond length in carbon monoxide (CO), which has a bond order of 1.
Conclusion:
In summary, there is an inverse relationship between bond order and bond length. As the bond order increases, the bond length decreases. This relationship is due to the increased electron sharing and electrostatic repulsion between electrons, resulting in a stronger bond and a shorter bond length.
Introduction:
Bond order is a measure of the strength or stability of a chemical bond between two atoms in a molecule. It indicates the number of electron pairs shared between two atoms. On the other hand, bond length refers to the distance between the nuclei of two bonded atoms.
Bond Order:
Bond order is determined by the number of bonding electrons divided by the number of bonding positions available. It provides information about the stability and strength of a bond. A higher bond order indicates a stronger bond and a shorter bond length.
Bond Length:
Bond length is influenced by several factors, including the size of the atoms involved, the number of bonding electrons, and the strength of the bond. A shorter bond length indicates a stronger bond.
The Relationship:
The relationship between bond order and bond length is inversely proportional. This means that as the bond order increases, the bond length decreases, and vice versa.
Explanation:
When two atoms form a bond, they share electrons to achieve a stable electron configuration. The more electron pairs shared between the two atoms, the stronger the bond becomes. As a result, the bond length decreases.
Electron Repulsion:
As more electrons are added to a bond, electrostatic repulsion between the electrons increases. This repulsion forces the bonded atoms closer together to minimize the repulsive forces.
Multiple Bonding:
In molecules with multiple bonds, such as double or triple bonds, the bond order is higher than in single bonds. This is because multiple bonds involve the sharing of more electron pairs. As a result, the bond length is shorter in multiple bonds compared to single bonds.
Example:
Let's consider the case of carbon dioxide (CO2). Each carbon-oxygen bond in CO2 is a double bond, meaning it has a bond order of 2. The carbon-oxygen bond length in CO2 is shorter than the carbon-oxygen bond length in carbon monoxide (CO), which has a bond order of 1.
Conclusion:
In summary, there is an inverse relationship between bond order and bond length. As the bond order increases, the bond length decreases. This relationship is due to the increased electron sharing and electrostatic repulsion between electrons, resulting in a stronger bond and a shorter bond length.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer?
Question Description
What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer?.
What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer?.
Solutions for What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice What do you think is the relationship between bond order and bond length?a)Directly proportionalb)Indirectly proportionalc)No relationd)Cannot predictCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















