Mechanical Engineering Exam > Mechanical Engineering Questions > 1 kg of water had been heated from 273 K to ...
Start Learning for Free
1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) is
Assume heating of water is a constant volume process.
Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgK
- a)97.7
- b)186.5
- c)112.4
- d)105.6
Correct answer is option 'A'. Can you explain this answer?
Most Upvoted Answer
1 kg of water had been heated from 273 K to 373 K by first bringing i...
Given data:
Mass of water (m) = 1 kg
Initial temperature (T1) = 273 K
Final temperature (T2) = 373 K
Temperature of first reservoir (T3) = 323 K
Temperature of second reservoir (T4) = 373 K
CV of water at 273 K (Cv1) = 4.217 kJ/kgK
CV of water at 323 K (Cv2) = 4.180 kJ/kgK
We can calculate the change in entropy of the universe (ΔS) by considering the entropy change of the water and the two reservoirs.
Entropy change of water:
ΔS_water = mCv ln(T2/T1)
Here, we assume that the heating of water is a constant volume process, so the heat supplied to the water is used only to increase its internal energy and temperature, and not to do any work.
Substituting the given values, we get:
ΔS_water = 1 * 4.217 * ln(373/273)
ΔS_water = 1 * 4.217 * 0.366
ΔS_water = 1.545 kJ/K
Entropy change of reservoirs:
ΔS_reservoir1 = -Q1/T3
ΔS_reservoir2 = -Q2/T4
Here, Q1 is the heat transferred from the first reservoir to the water, and Q2 is the heat transferred from the second reservoir to the water. Both these reservoirs are assumed to be large enough to maintain a constant temperature throughout the process.
The heat transferred from the first reservoir to the water can be calculated using the equation:
Q1 = mCv1(T3 - T1)
Substituting the given values, we get:
Q1 = 1 * 4.217 * (323 - 273)
Q1 = 211.7 kJ
Similarly, the heat transferred from the second reservoir to the water can be calculated using the equation:
Q2 = mCv2(T4 - T3)
Substituting the given values, we get:
Q2 = 1 * 4.180 * (373 - 323)
Q2 = 209.0 kJ
Substituting the values of Q1, Q2, T3 and T4, we get:
ΔS_reservoir1 = -211.7/323
ΔS_reservoir1 = -0.655 kJ/K
ΔS_reservoir2 = -209.0/373
ΔS_reservoir2 = -0.560 kJ/K
Total entropy change of the universe:
ΔS = ΔS_water + ΔS_reservoir1 + ΔS_reservoir2
ΔS = 1.545 - 0.655 - 0.560
ΔS = 0.330 kJ/K
Converting kJ/K to J/K, we get:
ΔS = 330 J/K
Therefore, the change in entropy of the universe is 97.7 J/K (approximately), which is closest to option A.
Mass of water (m) = 1 kg
Initial temperature (T1) = 273 K
Final temperature (T2) = 373 K
Temperature of first reservoir (T3) = 323 K
Temperature of second reservoir (T4) = 373 K
CV of water at 273 K (Cv1) = 4.217 kJ/kgK
CV of water at 323 K (Cv2) = 4.180 kJ/kgK
We can calculate the change in entropy of the universe (ΔS) by considering the entropy change of the water and the two reservoirs.
Entropy change of water:
ΔS_water = mCv ln(T2/T1)
Here, we assume that the heating of water is a constant volume process, so the heat supplied to the water is used only to increase its internal energy and temperature, and not to do any work.
Substituting the given values, we get:
ΔS_water = 1 * 4.217 * ln(373/273)
ΔS_water = 1 * 4.217 * 0.366
ΔS_water = 1.545 kJ/K
Entropy change of reservoirs:
ΔS_reservoir1 = -Q1/T3
ΔS_reservoir2 = -Q2/T4
Here, Q1 is the heat transferred from the first reservoir to the water, and Q2 is the heat transferred from the second reservoir to the water. Both these reservoirs are assumed to be large enough to maintain a constant temperature throughout the process.
The heat transferred from the first reservoir to the water can be calculated using the equation:
Q1 = mCv1(T3 - T1)
Substituting the given values, we get:
Q1 = 1 * 4.217 * (323 - 273)
Q1 = 211.7 kJ
Similarly, the heat transferred from the second reservoir to the water can be calculated using the equation:
Q2 = mCv2(T4 - T3)
Substituting the given values, we get:
Q2 = 1 * 4.180 * (373 - 323)
Q2 = 209.0 kJ
Substituting the values of Q1, Q2, T3 and T4, we get:
ΔS_reservoir1 = -211.7/323
ΔS_reservoir1 = -0.655 kJ/K
ΔS_reservoir2 = -209.0/373
ΔS_reservoir2 = -0.560 kJ/K
Total entropy change of the universe:
ΔS = ΔS_water + ΔS_reservoir1 + ΔS_reservoir2
ΔS = 1.545 - 0.655 - 0.560
ΔS = 0.330 kJ/K
Converting kJ/K to J/K, we get:
ΔS = 330 J/K
Therefore, the change in entropy of the universe is 97.7 J/K (approximately), which is closest to option A.
Free Test
FREE
| Start Free Test |
Community Answer
1 kg of water had been heated from 273 K to 373 K by first bringing i...
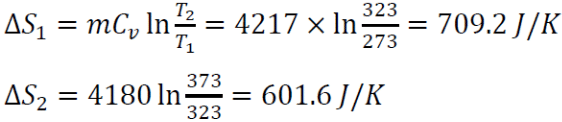
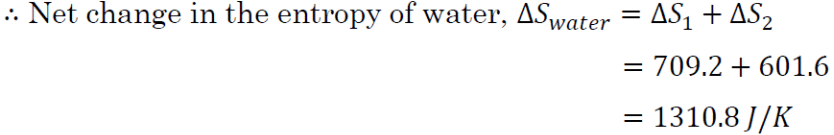
For the heat source, the change in entropy for stage 1,
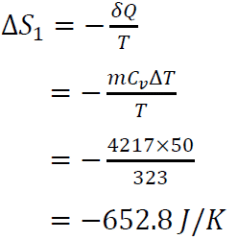
= - 652.8 J/K
For stage 2,
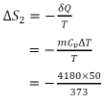
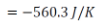
∴ Net change in the entropy of heat source,
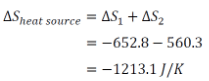
∴ Change in the entropy of universe
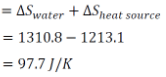

|
Explore Courses for Mechanical Engineering exam
|

|
Similar Mechanical Engineering Doubts
1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer?
Question Description
1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer?.
1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer?.
Solutions for 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer?, a detailed solution for 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice 1 kg of water had been heated from 273 K to 373 K by first bringing it in contact with a reservoir at 323 K and then with a reservoir at 373 K. The change in entropy of the universe (in J/K) isAssume heating of water is a constant volume process.Take CV of water at 273 K is 4.217 kJ/kgK and CV of water at 323 K is 4.180 kJ/kgKa)97.7b)186.5c)112.4d)105.6Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























