JEE Exam > JEE Questions > wo beaker are placed in a sealed flask. Beak...
Start Learning for Free
wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.
The molar mass of solute in solution B is closest to:
- a)60
- b)30
- c)181
- d)342
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
wo beaker are placed in a sealed flask. Beaker A initially contained ...
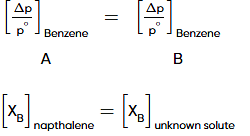
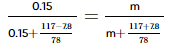
(m = moles of unknown solute)
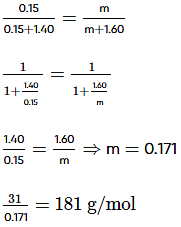
Free Test
FREE
| Start Free Test |
Community Answer
wo beaker are placed in a sealed flask. Beaker A initially contained ...
To solve this problem, we need to apply the concept of colligative properties and Raoult's law.
1. Understanding the concept of colligative properties:
Colligative properties are properties of a solution that depend on the number of solute particles, rather than the nature of the particles themselves. These properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
2. Applying Raoult's law:
Raoult's law states that the vapor pressure of a solvent above a solution is directly proportional to the mole fraction of the solvent present in the solution.
3. Analyzing the given information:
Beaker A initially contained 0.15 mol of naphthalene in 117 g of benzene. Since naphthalene is non-volatile, it does not contribute to the vapor pressure of the solution. Therefore, the vapor pressure of the solution is solely determined by the benzene.
Beaker B initially contained 31 g of an unknown compound in 117 g of benzene. The unknown compound is also non-volatile, so it does not contribute to the vapor pressure of the solution. Again, the vapor pressure of the solution is solely determined by the benzene.
4. Understanding the weight loss in beaker A:
At equilibrium, beaker A is found to have lost 7.8 g of weight. This weight loss can be attributed to the evaporation of benzene from the solution. As a result, the mole fraction of benzene in the solution decreases, leading to a decrease in its vapor pressure.
5. Calculating the mole fraction of benzene in beaker A:
To calculate the mole fraction of benzene in beaker A, we need to determine the moles of benzene present in the initial solution and the moles of benzene remaining in the solution after the weight loss.
The initial moles of benzene can be calculated using its molar mass:
Molar mass of benzene (C6H6) = 12.01 g/mol (carbon) + 1.01 g/mol (hydrogen) * 6 = 78.11 g/mol
Moles of benzene in beaker A = 117 g / 78.11 g/mol = 1.5 mol
The remaining moles of benzene can be calculated by subtracting the moles of naphthalene lost from the initial moles of benzene:
Remaining moles of benzene = 1.5 mol - 0.15 mol = 1.35 mol
Now, we can calculate the mole fraction of benzene in beaker A:
Mole fraction of benzene = Moles of benzene / Total moles of solute and solvent
Mole fraction of benzene = 1.35 mol / (0.15 mol + 1.35 mol) = 0.9
6. Applying Raoult's law to beaker B:
Since beaker B initially contained the unknown compound and benzene, and both are non-volatile, their mole fractions in the solution remain the same. Therefore, the mole fraction of benzene in beaker B is also 0.9.
7. Using Raoult's law to calculate the vapor pressure of benzene in beaker B:
According to Raoult's law, the vapor pressure of benzene above the solution is directly proportional to its mole fraction.
1. Understanding the concept of colligative properties:
Colligative properties are properties of a solution that depend on the number of solute particles, rather than the nature of the particles themselves. These properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
2. Applying Raoult's law:
Raoult's law states that the vapor pressure of a solvent above a solution is directly proportional to the mole fraction of the solvent present in the solution.
3. Analyzing the given information:
Beaker A initially contained 0.15 mol of naphthalene in 117 g of benzene. Since naphthalene is non-volatile, it does not contribute to the vapor pressure of the solution. Therefore, the vapor pressure of the solution is solely determined by the benzene.
Beaker B initially contained 31 g of an unknown compound in 117 g of benzene. The unknown compound is also non-volatile, so it does not contribute to the vapor pressure of the solution. Again, the vapor pressure of the solution is solely determined by the benzene.
4. Understanding the weight loss in beaker A:
At equilibrium, beaker A is found to have lost 7.8 g of weight. This weight loss can be attributed to the evaporation of benzene from the solution. As a result, the mole fraction of benzene in the solution decreases, leading to a decrease in its vapor pressure.
5. Calculating the mole fraction of benzene in beaker A:
To calculate the mole fraction of benzene in beaker A, we need to determine the moles of benzene present in the initial solution and the moles of benzene remaining in the solution after the weight loss.
The initial moles of benzene can be calculated using its molar mass:
Molar mass of benzene (C6H6) = 12.01 g/mol (carbon) + 1.01 g/mol (hydrogen) * 6 = 78.11 g/mol
Moles of benzene in beaker A = 117 g / 78.11 g/mol = 1.5 mol
The remaining moles of benzene can be calculated by subtracting the moles of naphthalene lost from the initial moles of benzene:
Remaining moles of benzene = 1.5 mol - 0.15 mol = 1.35 mol
Now, we can calculate the mole fraction of benzene in beaker A:
Mole fraction of benzene = Moles of benzene / Total moles of solute and solvent
Mole fraction of benzene = 1.35 mol / (0.15 mol + 1.35 mol) = 0.9
6. Applying Raoult's law to beaker B:
Since beaker B initially contained the unknown compound and benzene, and both are non-volatile, their mole fractions in the solution remain the same. Therefore, the mole fraction of benzene in beaker B is also 0.9.
7. Using Raoult's law to calculate the vapor pressure of benzene in beaker B:
According to Raoult's law, the vapor pressure of benzene above the solution is directly proportional to its mole fraction.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer?
Question Description
wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer?.
wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer?.
Solutions for wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer?, a detailed solution for wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice wo beaker are placed in a sealed flask. Beaker A initially contained 0.15 mol of naphthalene (non-volatile) in 117 g of benzene and beaker B initially contained 31 g of an unknown compound (non-volatile, non-electrolytic) in 117 g of benzene. At equilibrium, beaker A is found to have lost 7.8 g of weight. Assume ideal behaviour of both solutions to answer the following question.The molar mass of solute in solution B is closest to:a)60b)30c)181d)342Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























