JEE Exam > JEE Questions > An auto catalytic reaction is represented as...
Start Learning for Free
An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-
- a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reaction
- b)In an autocatalytic reaction product form a complex of lower activation energies.
- c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3
- d)The overall order of the above reaction is three
Correct answer is option 'A,C'. Can you explain this answer?
Most Upvoted Answer
An auto catalytic reaction is represented as A + B ⟶ D Where initial ...
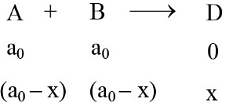
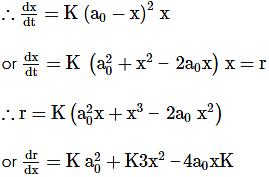
∴ at the maximum rate, dr/dx = 0
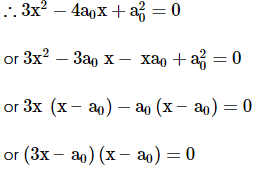
∴ x = a0 or x = a0/3
But x ≠ a0 ∴ x = a0/3
Free Test
FREE
| Start Free Test |
Community Answer
An auto catalytic reaction is represented as A + B ⟶ D Where initial ...
An autocatalytic reaction is a type of reaction where the product itself acts as a catalyst, meaning it increases the rate of the reaction. Let's analyze the given options to understand why options A and C are correct.
a) An autocatalytic reaction is the such reaction in which product itself increases the speed of reaction.
In an autocatalytic reaction, the product formed in the reaction acts as a catalyst, meaning it increases the rate of the reaction. In the given reaction, the product D is being formed, and if it acts as a catalyst, it will increase the speed of the reaction. Therefore, option A is correct.
c) The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3.
To determine the maximum rate of the reaction, we need to find the concentration of D at which the rate is highest. Since we are given that the initial concentration of D is zero, the concentration of D will increase as the reaction proceeds. As the concentration of D increases, it will act as a catalyst and enhance the rate of the reaction. However, as the concentration of D continues to increase, it will reach a point where the rate of formation of D will start to decrease due to the depletion of reactants A and B. This maximum rate is obtained when the concentration of D is equal to a0/3. Therefore, option C is correct.
b) In an autocatalytic reaction, the product forms a complex of lower activation energies.
This statement is not necessarily true for all autocatalytic reactions. While it is possible for the product to form a complex of lower activation energies in some autocatalytic reactions, it is not a general characteristic of autocatalysis. Therefore, option B is incorrect.
d) The overall order of the above reaction is three.
The overall order of a reaction is the sum of the powers to which the concentration terms are raised in the rate equation. From the given reaction, we can see that the rate of the reaction depends on the concentrations of A and B. However, there is no information given about the specific rate equation or the powers to which the concentration terms are raised. Therefore, we cannot determine the overall order of the reaction from the given information. Therefore, option D is incorrect.
In summary, options A and C are the correct statements regarding autocatalytic reactions. The product of an autocatalytic reaction increases the speed of the reaction, and the maximum rate is obtained when the concentration of the product is a0/3.
a) An autocatalytic reaction is the such reaction in which product itself increases the speed of reaction.
In an autocatalytic reaction, the product formed in the reaction acts as a catalyst, meaning it increases the rate of the reaction. In the given reaction, the product D is being formed, and if it acts as a catalyst, it will increase the speed of the reaction. Therefore, option A is correct.
c) The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3.
To determine the maximum rate of the reaction, we need to find the concentration of D at which the rate is highest. Since we are given that the initial concentration of D is zero, the concentration of D will increase as the reaction proceeds. As the concentration of D increases, it will act as a catalyst and enhance the rate of the reaction. However, as the concentration of D continues to increase, it will reach a point where the rate of formation of D will start to decrease due to the depletion of reactants A and B. This maximum rate is obtained when the concentration of D is equal to a0/3. Therefore, option C is correct.
b) In an autocatalytic reaction, the product forms a complex of lower activation energies.
This statement is not necessarily true for all autocatalytic reactions. While it is possible for the product to form a complex of lower activation energies in some autocatalytic reactions, it is not a general characteristic of autocatalysis. Therefore, option B is incorrect.
d) The overall order of the above reaction is three.
The overall order of a reaction is the sum of the powers to which the concentration terms are raised in the rate equation. From the given reaction, we can see that the rate of the reaction depends on the concentrations of A and B. However, there is no information given about the specific rate equation or the powers to which the concentration terms are raised. Therefore, we cannot determine the overall order of the reaction from the given information. Therefore, option D is incorrect.
In summary, options A and C are the correct statements regarding autocatalytic reactions. The product of an autocatalytic reaction increases the speed of the reaction, and the maximum rate is obtained when the concentration of the product is a0/3.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer?
Question Description
An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer?.
An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer?.
Solutions for An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer?, a detailed solution for An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? has been provided alongside types of An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice An auto catalytic reaction is represented as A + B ⟶ D Where initial concentrations of A and B Each equal to a0. If initial concentration of D Is zero-a)An autocatalytic reaction is the such reaction in which product itself increases the speed of reactionb)In an autocatalytic reaction product form a complex of lower activation energies.c)The maximum rate for the above autocatalytic reaction is obtained when [D] = a0/3d)The overall order of the above reaction is threeCorrect answer is option 'A,C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























