Civil Engineering (CE) Exam > Civil Engineering (CE) Questions > If one of the ends of the glass capillary tub...
Start Learning for Free
If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:
- a)liquid would rise in the capillary
- b)liquid can rise or fall depending on density
- c)liquid would remain at same level in the capillary as free surface
- d)liquid would fall in the capillary
Correct answer is option 'A'. Can you explain this answer?
Most Upvoted Answer
If one of the ends of the glass capillary tube is immersed in a liquid...
Capillary action:
- It is the ability of a fluid to flow through a narrow space without the application of an external force.
- When a tube of very small diameter is dipped inside a fluid, we can see either a rise or a fall of fluid inside a tube.
- If the fluid level increases in the tube it is called capillary rise and if the fluid level decreases it is called capillary fall.
- The reason for the capillary action is adhesive and cohesive forces.
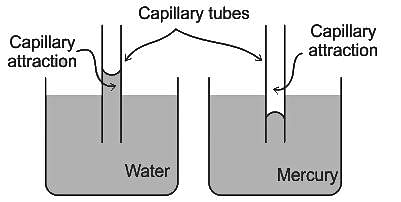
- If the adhesive force is more compared to cohesive force, then there will be the capillary rise and the meniscus will be of concave shape.
- If the cohesive force is more compared to adhesive force, then there will be capillary fall and the meniscus will be of convex shape.
- For capillary rise the angle of contact is less than 90° and for capillary fall the angle of contact is greater than 90°.
Free Test
FREE
| Start Free Test |
Community Answer
If one of the ends of the glass capillary tube is immersed in a liquid...
Explanation:
Capillary Action:
Capillary action is the ability of a liquid to flow in narrow spaces without the assistance of, or even in opposition to, external forces like gravity. This phenomenon is due to the combination of adhesive and cohesive forces within the liquid.
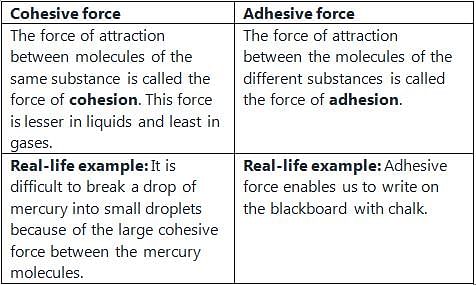
Adhesion and Cohesion:
Adhesion is the attraction between molecules of different substances, while cohesion is the attraction between molecules of the same substance. When the adhesive forces between the liquid and the walls of the capillary tube are stronger than the cohesive forces within the liquid, capillary action occurs.
Effect of Adhesion on Capillary Action:
When one end of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion, the liquid molecules are attracted to the walls of the tube. This causes the liquid to rise in the capillary tube against the force of gravity. The higher the adhesion between the liquid and the tube, the higher the liquid will rise in the capillary.
Therefore, in this scenario, the liquid would rise in the capillary tube due to the stronger adhesion forces pulling the liquid up the tube.
Capillary Action:
Capillary action is the ability of a liquid to flow in narrow spaces without the assistance of, or even in opposition to, external forces like gravity. This phenomenon is due to the combination of adhesive and cohesive forces within the liquid.
Adhesion and Cohesion:
Adhesion is the attraction between molecules of different substances, while cohesion is the attraction between molecules of the same substance. When the adhesive forces between the liquid and the walls of the capillary tube are stronger than the cohesive forces within the liquid, capillary action occurs.
Effect of Adhesion on Capillary Action:
When one end of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion, the liquid molecules are attracted to the walls of the tube. This causes the liquid to rise in the capillary tube against the force of gravity. The higher the adhesion between the liquid and the tube, the higher the liquid will rise in the capillary.
Therefore, in this scenario, the liquid would rise in the capillary tube due to the stronger adhesion forces pulling the liquid up the tube.

|
Explore Courses for Civil Engineering (CE) exam
|

|
Similar Civil Engineering (CE) Doubts
If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer?
Question Description
If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? for Civil Engineering (CE) 2025 is part of Civil Engineering (CE) preparation. The Question and answers have been prepared according to the Civil Engineering (CE) exam syllabus. Information about If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer?.
If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? for Civil Engineering (CE) 2025 is part of Civil Engineering (CE) preparation. The Question and answers have been prepared according to the Civil Engineering (CE) exam syllabus. Information about If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer?.
Solutions for If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Civil Engineering (CE).
Download more important topics, notes, lectures and mock test series for Civil Engineering (CE) Exam by signing up for free.
Here you can find the meaning of If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If one of the ends of the glass capillary tube is immersed in a liquid with stronger adhesion than cohesion then:a)liquid would rise in the capillaryb)liquid can rise or fall depending on densityc)liquid would remain at same level in the capillary as free surfaced)liquid would fall in the capillaryCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Civil Engineering (CE) tests.

|
Explore Courses for Civil Engineering (CE) exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























