JEE Exam > JEE Questions > The compound [Co(H2O)6]2+, having three unpai...
Start Learning for Free
The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because of
- a)an increase in the number of unpaired electrons
- b)some contribution of the total orbital motion of the electron to the magnetic moment
- c)change in the orbital spin of the electron
- d)d-d* transitions
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
The compound [Co(H2O)6]2+, having three unpaired electrons, shows the ...
From Quantum Mechanics viewpoint, the magnetic moment is dependent on both spin and orbital angular momentum contributions. The spin-only formula used is given by
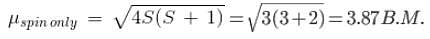
And this can be modified to include the orbital angular momentum as follows.
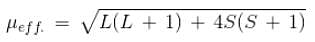
Therefore, the value of the magnetic moment can differ because of the contribution of the total orbital motion of the electron to the magnetic moment. Thus, this is the correct answer.
Free Test
FREE
| Start Free Test |
Community Answer
The compound [Co(H2O)6]2+, having three unpaired electrons, shows the ...
Explanation:
Contribution of orbital motion to magnetic moment:
- The discrepancy between the experimental and theoretical magnetic moments of [Co(H2O)6]2+ can be attributed to the contribution of the orbital motion of the electrons to the magnetic moment.
- The theoretical calculated value only considers the spin magnetic moment of the unpaired electrons but does not take into account the orbital motion contribution.
Effect of orbital motion on magnetic moment:
- The orbital motion of an electron around the nucleus generates an orbital magnetic moment.
- This orbital magnetic moment interacts with the external magnetic field and contributes to the overall magnetic moment of the complex.
- In the case of [Co(H2O)6]2+, the presence of three unpaired electrons with orbital motion contributes to the experimental magnetic moment exceeding the theoretical value.
Reason for discrepancy:
- The discrepancy arises because the theoretical calculation typically focuses only on the spin magnetic moment of the unpaired electrons, neglecting the contribution from orbital motion.
- When the orbital motion contribution is considered, the overall magnetic moment of the complex increases, leading to the observed deviation between the experimental and theoretical values.
Conclusion:
- In summary, the discrepancy in the magnetic moment of [Co(H2O)6]2+ can be explained by the contribution of the orbital motion of the electrons to the magnetic moment, which is not accounted for in the theoretical calculation.
Contribution of orbital motion to magnetic moment:
- The discrepancy between the experimental and theoretical magnetic moments of [Co(H2O)6]2+ can be attributed to the contribution of the orbital motion of the electrons to the magnetic moment.
- The theoretical calculated value only considers the spin magnetic moment of the unpaired electrons but does not take into account the orbital motion contribution.
Effect of orbital motion on magnetic moment:
- The orbital motion of an electron around the nucleus generates an orbital magnetic moment.
- This orbital magnetic moment interacts with the external magnetic field and contributes to the overall magnetic moment of the complex.
- In the case of [Co(H2O)6]2+, the presence of three unpaired electrons with orbital motion contributes to the experimental magnetic moment exceeding the theoretical value.
Reason for discrepancy:
- The discrepancy arises because the theoretical calculation typically focuses only on the spin magnetic moment of the unpaired electrons, neglecting the contribution from orbital motion.
- When the orbital motion contribution is considered, the overall magnetic moment of the complex increases, leading to the observed deviation between the experimental and theoretical values.
Conclusion:
- In summary, the discrepancy in the magnetic moment of [Co(H2O)6]2+ can be explained by the contribution of the orbital motion of the electrons to the magnetic moment, which is not accounted for in the theoretical calculation.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer?
Question Description
The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer?.
The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer?.
Solutions for The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The compound [Co(H2O)6]2+, having three unpaired electrons, shows the experimental magnetic moment of 4.40 BM, while the theoretical calculated value is 3.87 BM. This is because ofa)an increase in the number of unpaired electronsb)some contribution of the total orbital motion of the electron to the magnetic momentc)change in the orbital spin of the electrond)d-d* transitionsCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























