JEE Exam > JEE Questions > How do you account for the following observat...
Start Learning for Free
How do you account for the following observations ?
Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.
Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.
- a)In acidic conditions the reactants form a homogeneous medium
- b)In alkaline / neutral medium the reactants do not exist as heterogeneous medium
- c)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is faster
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
How do you account for the following observations ?Though alkaline pot...
Oxidation of toluene to benzoic acid in acidic medium
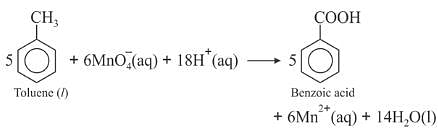
Oxidation of toluene to benzoic acid in basic and neutral medium
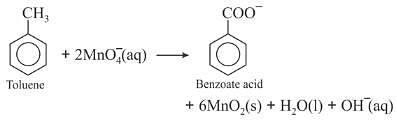
On industrial scale, alcoholic potassium permanganate is preferred to acidic or alkaline potassium permanganate because in the presence of alcohol both the reactants KMnO4 and C6H5CH3 are mixed very well and form homogeneous solution and in homogeneous medium reaction takes place faster than in heterogeneous medium. Further more in neutral medium OH- ions are produced in the reaction itself.
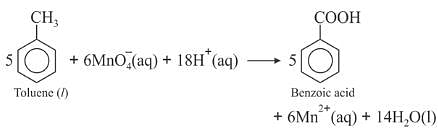
Oxidation of toluene to benzoic acid in basic and neutral medium

On industrial scale, alcoholic potassium permanganate is preferred to acidic or alkaline potassium permanganate because in the presence of alcohol both the reactants KMnO4 and C6H5CH3 are mixed very well and form homogeneous solution and in homogeneous medium reaction takes place faster than in heterogeneous medium. Further more in neutral medium OH- ions are produced in the reaction itself.
Free Test
FREE
| Start Free Test |
Community Answer
How do you account for the following observations ?Though alkaline pot...
Explanation:
Alcoholic potassium permanganate (KMnO4) is used as an oxidant in the manufacture of benzoic acid from toluene because it provides a suitable medium for the reaction to occur efficiently. This is due to the following observations:
1. Homogeneous Medium: Alcoholic KMnO4 forms a homogeneous medium with the reactants. In a homogeneous medium, all the reactants and the oxidant are present in the same phase, which allows for easier and faster reaction kinetics. This is because the oxidant molecules can easily come in contact with the reactant molecules, leading to a higher rate of reaction.
2. Faster Reaction: The reaction between toluene and KMnO4 in alcoholic medium is faster compared to acidic or alkaline conditions. This is because the alcoholic medium enhances the reactivity of both the oxidant and the reactant. The presence of alcohol molecules facilitates the breaking of bonds in the reactant molecules and promotes the transfer of electrons during the oxidation process.
3. Oxidation Potential: Alcoholic KMnO4 has a higher oxidation potential compared to alkaline or acidic KMnO4. This means that it is a stronger oxidizing agent and is more effective in oxidizing the reactant (toluene) to form the desired product (benzoic acid). The higher oxidation potential of alcoholic KMnO4 is attributed to the presence of alcohol molecules, which can act as electron donors and enhance the reactivity of the oxidant.
Redox Equation:
The balanced redox equation for the reaction between toluene and alcoholic KMnO4 can be written as follows:
C6H5CH3 + 2KMnO4 + 3H2O → C6H5COOH + 2MnO2 + 2KOH + 4H2O
In this equation, toluene (C6H5CH3) is oxidized to benzoic acid (C6H5COOH) by the alcoholic KMnO4. The MnO4- ions in KMnO4 are reduced to MnO2, and water (H2O) is used as a solvent and a source of protons (H+) in the reaction.
Overall, the use of alcoholic KMnO4 as an oxidant in the manufacture of benzoic acid from toluene offers several advantages, including a homogeneous reaction medium, faster reaction kinetics, and a higher oxidation potential. These factors contribute to the efficiency and effectiveness of the oxidation process.
Alcoholic potassium permanganate (KMnO4) is used as an oxidant in the manufacture of benzoic acid from toluene because it provides a suitable medium for the reaction to occur efficiently. This is due to the following observations:
1. Homogeneous Medium: Alcoholic KMnO4 forms a homogeneous medium with the reactants. In a homogeneous medium, all the reactants and the oxidant are present in the same phase, which allows for easier and faster reaction kinetics. This is because the oxidant molecules can easily come in contact with the reactant molecules, leading to a higher rate of reaction.
2. Faster Reaction: The reaction between toluene and KMnO4 in alcoholic medium is faster compared to acidic or alkaline conditions. This is because the alcoholic medium enhances the reactivity of both the oxidant and the reactant. The presence of alcohol molecules facilitates the breaking of bonds in the reactant molecules and promotes the transfer of electrons during the oxidation process.
3. Oxidation Potential: Alcoholic KMnO4 has a higher oxidation potential compared to alkaline or acidic KMnO4. This means that it is a stronger oxidizing agent and is more effective in oxidizing the reactant (toluene) to form the desired product (benzoic acid). The higher oxidation potential of alcoholic KMnO4 is attributed to the presence of alcohol molecules, which can act as electron donors and enhance the reactivity of the oxidant.
Redox Equation:
The balanced redox equation for the reaction between toluene and alcoholic KMnO4 can be written as follows:
C6H5CH3 + 2KMnO4 + 3H2O → C6H5COOH + 2MnO2 + 2KOH + 4H2O
In this equation, toluene (C6H5CH3) is oxidized to benzoic acid (C6H5COOH) by the alcoholic KMnO4. The MnO4- ions in KMnO4 are reduced to MnO2, and water (H2O) is used as a solvent and a source of protons (H+) in the reaction.
Overall, the use of alcoholic KMnO4 as an oxidant in the manufacture of benzoic acid from toluene offers several advantages, including a homogeneous reaction medium, faster reaction kinetics, and a higher oxidation potential. These factors contribute to the efficiency and effectiveness of the oxidation process.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer?
Question Description
How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer?.
How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer?.
Solutions for How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice How do you account for the following observations ?Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why ? Write a balance redox equation for the reaction.a)In acidic conditions the reactants form a homogeneous mediumb)In alkaline / neutral medium the reactants do not exist as heterogeneous mediumc)In alcoholic KMnO4, the reactants form a homogeneous medium and reaction is fasterd)None of theseCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























