Chemistry Exam > Chemistry Questions > The rotational spectrum of HF has lines which...
Start Learning for Free
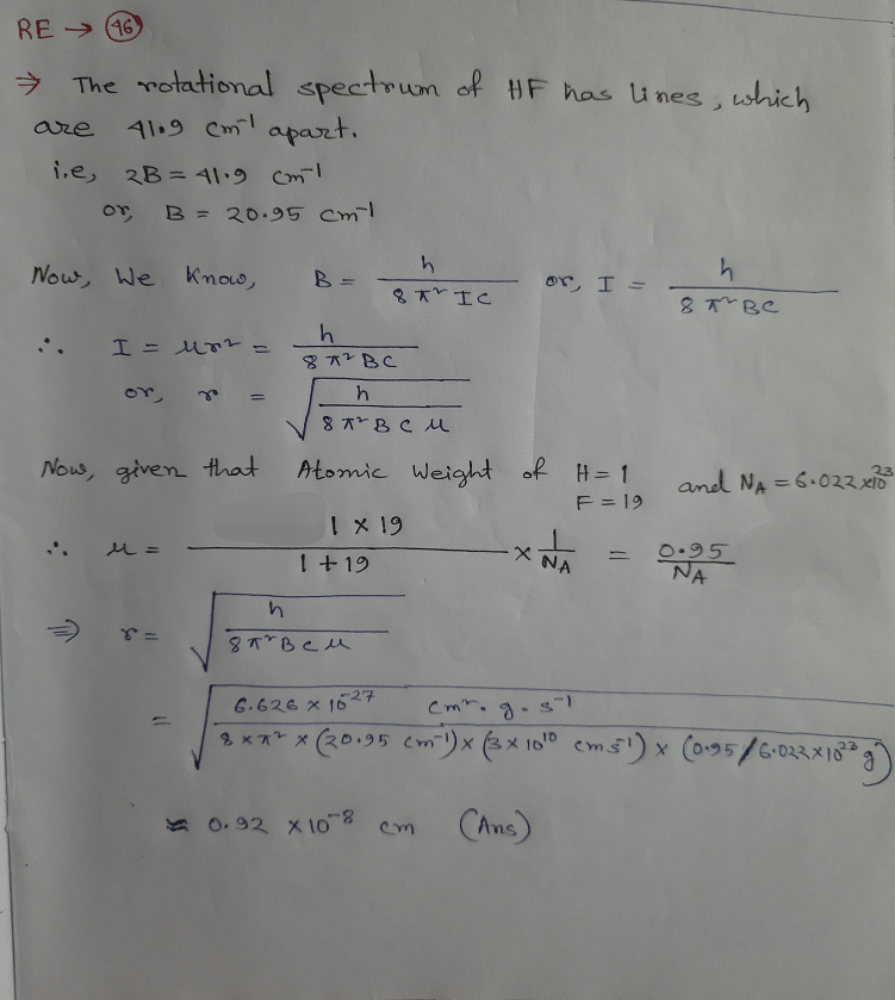
The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)?
Most Upvoted Answer
The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalcul...
**Bond Length Calculation for HF**
To calculate the bond length in HF using the given rotational spectrum data, we can utilize the formula:
ΔE = B(J+1) - B(J)
Where:
ΔE is the energy difference between two rotational levels (in this case, the spacing between lines)
B is the rotational constant
J is the rotational quantum number
**Calculating the Rotational Constant**
The rotational constant (B) can be determined using the formula:
B = h/(8π²cI)
Where:
h is Planck's constant (6.626 x 10^-34 J·s)
c is the speed of light (3.00 x 10^8 m/s)
I is the reduced mass of the molecule
The reduced mass (μ) can be calculated using the formula:
μ = (m1 * m2) / (m1 + m2)
Where:
m1 and m2 are the masses of hydrogen and fluorine atoms, respectively
Given that the atomic weights of H and F are 1 and 19, respectively, and Avogadro's number (N₄) is 6.022 x 10^23 mol⁻¹, we can calculate the masses of H and F atoms:
m₁ = (1 g/mol) / (6.022 x 10²³ atoms/mol) = 1.66 x 10⁻²⁴ g
m₂ = (19 g/mol) / (6.022 x 10²³ atoms/mol) = 3.15 x 10⁻²³ g
Substituting these values into the reduced mass equation:
μ = (1.66 x 10⁻²⁴ g * 3.15 x 10⁻²³ g) / (1.66 x 10⁻²⁴ g + 3.15 x 10⁻²³ g)
= 3.03 x 10⁻²⁶ g
**Calculating the Bond Length**
Now, we can calculate the rotational constant (B) using the reduced mass:
B = (6.626 x 10^-34 J·s) / (8π² * 3.00 x 10^8 m/s * 3.03 x 10⁻²⁶ g)
= 1.01 x 10^-10 J
Given that the spacing between lines in the rotational spectrum of HF is 41.9 cm⁻¹, which is equivalent to 1.25 x 10^10 Hz, we can rearrange the formula for energy difference:
ΔE = B(J+1) - B(J)
Solving for J+1 and J:
J+1 = ΔE / B + J
J = J+1 - 1
Substituting the values:
J+1 = (1.25 x 10^10 Hz) / (1.01 x 10^-10 J) + J
J = (1.25 x 10^10 Hz) / (1.01 x 10^-10 J) - 1
J ≈ 137
The rotational quantum number (J) represents the maximum value of the angular momentum of the molecule. Since J is very high, it indicates that the molecule has a large moment of inertia and is relatively heavy.
**Spacing
To calculate the bond length in HF using the given rotational spectrum data, we can utilize the formula:
ΔE = B(J+1) - B(J)
Where:
ΔE is the energy difference between two rotational levels (in this case, the spacing between lines)
B is the rotational constant
J is the rotational quantum number
**Calculating the Rotational Constant**
The rotational constant (B) can be determined using the formula:
B = h/(8π²cI)
Where:
h is Planck's constant (6.626 x 10^-34 J·s)
c is the speed of light (3.00 x 10^8 m/s)
I is the reduced mass of the molecule
The reduced mass (μ) can be calculated using the formula:
μ = (m1 * m2) / (m1 + m2)
Where:
m1 and m2 are the masses of hydrogen and fluorine atoms, respectively
Given that the atomic weights of H and F are 1 and 19, respectively, and Avogadro's number (N₄) is 6.022 x 10^23 mol⁻¹, we can calculate the masses of H and F atoms:
m₁ = (1 g/mol) / (6.022 x 10²³ atoms/mol) = 1.66 x 10⁻²⁴ g
m₂ = (19 g/mol) / (6.022 x 10²³ atoms/mol) = 3.15 x 10⁻²³ g
Substituting these values into the reduced mass equation:
μ = (1.66 x 10⁻²⁴ g * 3.15 x 10⁻²³ g) / (1.66 x 10⁻²⁴ g + 3.15 x 10⁻²³ g)
= 3.03 x 10⁻²⁶ g
**Calculating the Bond Length**
Now, we can calculate the rotational constant (B) using the reduced mass:
B = (6.626 x 10^-34 J·s) / (8π² * 3.00 x 10^8 m/s * 3.03 x 10⁻²⁶ g)
= 1.01 x 10^-10 J
Given that the spacing between lines in the rotational spectrum of HF is 41.9 cm⁻¹, which is equivalent to 1.25 x 10^10 Hz, we can rearrange the formula for energy difference:
ΔE = B(J+1) - B(J)
Solving for J+1 and J:
J+1 = ΔE / B + J
J = J+1 - 1
Substituting the values:
J+1 = (1.25 x 10^10 Hz) / (1.01 x 10^-10 J) + J
J = (1.25 x 10^10 Hz) / (1.01 x 10^-10 J) - 1
J ≈ 137
The rotational quantum number (J) represents the maximum value of the angular momentum of the molecule. Since J is very high, it indicates that the molecule has a large moment of inertia and is relatively heavy.
**Spacing
Community Answer
The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalcul...

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)?
Question Description
The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)?.
The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)?.
Solutions for The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? defined & explained in the simplest way possible. Besides giving the explanation of
The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)?, a detailed solution for The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? has been provided alongside types of The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? theory, EduRev gives you an
ample number of questions to practice The rotational spectrum of HF has lines which are 41.9 cm¹ apartCalculate the bond length in HF[Given: AtWtof H= 1 . F = 19 , N_{4} = 6.022 * 10 ^ 2 mol-']What will be the spacing between lines if DF was investigated? [D = 2] (Assume the bond length to remain unaltered upon isotopic substitution.)? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















