BPSC (Bihar) Exam > BPSC (Bihar) Questions > Which of the following properties of water ca...
Start Learning for Free
Which of the following properties of water can be used to explain the spherical shape of rain droplets?
- a)Surface tension
- b)Critical phenomenon
- c)Viscosity
- d)Pressure
- e)None of the above/ More than one of the above
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Which of the following properties of water can be used to explain the ...
Explanation:
The correct answer is option 'A' - Surface tension.
Surface Tension:
Surface tension is a property of liquids that arises due to the cohesive forces between the molecules at the surface of the liquid. It is the force that acts parallel to the surface and tends to minimize the surface area of a liquid.
Explanation:
When raindrops fall through the atmosphere, they experience resistance from the air. This resistance causes the raindrop to deform slightly, and surface tension tries to minimize the surface area of the droplet. As a result, the raindrop takes on a spherical shape.
Surface tension and spherical shape:
- Surface tension acts in such a way that it minimizes the surface area of a liquid. In the case of a raindrop, surface tension pulls the molecules at the surface of the droplet towards each other, causing the droplet to take on a spherical shape.
- The spherical shape is the most efficient shape for minimizing the surface area of a given volume. A sphere has the smallest surface area for a given volume compared to any other shape.
- Due to the cohesive forces between the water molecules, the surface tension in a raindrop is strong enough to overcome the resistance from the air and pull the droplet into a spherical shape.
Other properties:
- Critical phenomenon: Critical phenomenon refers to the behavior of a substance at its critical point, where its properties change dramatically. This property does not directly explain the spherical shape of rain droplets.
- Viscosity: Viscosity is a measure of a liquid's resistance to flow. While viscosity affects the behavior of liquids, it does not directly explain the spherical shape of rain droplets.
- Pressure: Pressure can affect the size and shape of rain droplets but does not specifically explain the spherical shape. Pressure variations in the atmosphere can cause the droplets to grow or shrink, but the underlying force responsible for the spherical shape is surface tension.
Conclusion:
The spherical shape of rain droplets can be explained by the property of surface tension. Surface tension acts to minimize the surface area of the droplet, resulting in a spherical shape that is the most efficient for minimizing surface area.
The correct answer is option 'A' - Surface tension.
Surface Tension:
Surface tension is a property of liquids that arises due to the cohesive forces between the molecules at the surface of the liquid. It is the force that acts parallel to the surface and tends to minimize the surface area of a liquid.
Explanation:
When raindrops fall through the atmosphere, they experience resistance from the air. This resistance causes the raindrop to deform slightly, and surface tension tries to minimize the surface area of the droplet. As a result, the raindrop takes on a spherical shape.
Surface tension and spherical shape:
- Surface tension acts in such a way that it minimizes the surface area of a liquid. In the case of a raindrop, surface tension pulls the molecules at the surface of the droplet towards each other, causing the droplet to take on a spherical shape.
- The spherical shape is the most efficient shape for minimizing the surface area of a given volume. A sphere has the smallest surface area for a given volume compared to any other shape.
- Due to the cohesive forces between the water molecules, the surface tension in a raindrop is strong enough to overcome the resistance from the air and pull the droplet into a spherical shape.
Other properties:
- Critical phenomenon: Critical phenomenon refers to the behavior of a substance at its critical point, where its properties change dramatically. This property does not directly explain the spherical shape of rain droplets.
- Viscosity: Viscosity is a measure of a liquid's resistance to flow. While viscosity affects the behavior of liquids, it does not directly explain the spherical shape of rain droplets.
- Pressure: Pressure can affect the size and shape of rain droplets but does not specifically explain the spherical shape. Pressure variations in the atmosphere can cause the droplets to grow or shrink, but the underlying force responsible for the spherical shape is surface tension.
Conclusion:
The spherical shape of rain droplets can be explained by the property of surface tension. Surface tension acts to minimize the surface area of the droplet, resulting in a spherical shape that is the most efficient for minimizing surface area.
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following properties of water can be used to explain the ...
Surface tension:
- Surface tension is the property by virtue of which a liquid tries to minimize its free surface area.
- In a spherical shape, the surface area is minimum and for this reason, the raindrops are spherical.
- Surface tension is measured as the force acting per unit length of an imaginary line drawn on the liquid surface.
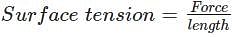
Two factors can affect the surface tension of a liquid. The factors are-
- Temperature: If temperature increases then the surface tension of a liquid decreases.
- Soluble Impurities: In the case of less soluble impurities, the surface tension decreases. But, for highly soluble impurities in the liquid the surface tension increases.
Attention BPSC (Bihar) Students!
To make sure you are not studying endlessly, EduRev has designed BPSC (Bihar) study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in BPSC (Bihar).

|
Explore Courses for BPSC (Bihar) exam
|

|
Similar BPSC (Bihar) Doubts
Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer?
Question Description
Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? for BPSC (Bihar) 2024 is part of BPSC (Bihar) preparation. The Question and answers have been prepared according to the BPSC (Bihar) exam syllabus. Information about Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for BPSC (Bihar) 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer?.
Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? for BPSC (Bihar) 2024 is part of BPSC (Bihar) preparation. The Question and answers have been prepared according to the BPSC (Bihar) exam syllabus. Information about Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for BPSC (Bihar) 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for BPSC (Bihar).
Download more important topics, notes, lectures and mock test series for BPSC (Bihar) Exam by signing up for free.
Here you can find the meaning of Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following properties of water can be used to explain the spherical shape of rain droplets?a)Surface tensionb)Critical phenomenonc)Viscosityd)Pressuree)None of the above/ More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice BPSC (Bihar) tests.

|
Explore Courses for BPSC (Bihar) exam
|

|
Suggested Free Tests
Test: Human Environment Interactions the Tropical and The Subtropical Region
Test | 15 questions
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























