JEE Exam > JEE Questions > Given that the N shell of an element contains...
Start Learning for Free
Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.?
Verified Answer
Given that the N shell of an element contains 10 electrons,write the e...
Ans.
Method to Solve :
Nickel 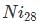 has 28 electrons. 18 electrons fill up the third electron shell leaving 10 valance electrons. 2 electrons in the 4s and 8 elections in the 3d.
has 28 electrons. 18 electrons fill up the third electron shell leaving 10 valance electrons. 2 electrons in the 4s and 8 elections in the 3d.
When Nickel becomes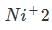 Nickel has lost 2 electrons leaving the atom with only 8 valance electrons.
Nickel has lost 2 electrons leaving the atom with only 8 valance electrons.
The 4s electrons a lower energy level that the 3d electrons because of the simpler electron path of the S orbital. So in the ground state the electrons being lost should be the 3d electrons.
However the 4s electrons are further from the nucleus so losing the the 2 4s electrons leaves only the third shell electrons making the atoms more stable than losing the 3d electrons.
So the somewhat stable electron configuration of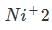 is
is 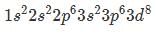
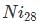 has 28 electrons. 18 electrons fill up the third electron shell leaving 10 valance electrons. 2 electrons in the 4s and 8 elections in the 3d.
has 28 electrons. 18 electrons fill up the third electron shell leaving 10 valance electrons. 2 electrons in the 4s and 8 elections in the 3d.When Nickel becomes
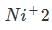 Nickel has lost 2 electrons leaving the atom with only 8 valance electrons.
Nickel has lost 2 electrons leaving the atom with only 8 valance electrons.The 4s electrons a lower energy level that the 3d electrons because of the simpler electron path of the S orbital. So in the ground state the electrons being lost should be the 3d electrons.
However the 4s electrons are further from the nucleus so losing the the 2 4s electrons leaves only the third shell electrons making the atoms more stable than losing the 3d electrons.
So the somewhat stable electron configuration of
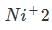 is
is 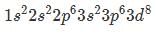
 This question is part of UPSC exam. View all JEE courses
This question is part of UPSC exam. View all JEE courses
Most Upvoted Answer
Given that the N shell of an element contains 10 electrons,write the e...
Electronic Configuration of the Stable Ion with 10 Electrons in the N Shell
To determine the electronic configuration of the stable ion with 10 electrons in the N shell, we need to understand the rules governing electron filling in atomic orbitals.
1. Aufbau Principle:
The Aufbau principle states that electrons fill the lowest energy orbitals first before moving to higher energy orbitals. Therefore, we begin by filling the lower energy orbitals before moving to the higher ones.
2. Pauli Exclusion Principle:
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can accommodate a maximum of two electrons with opposite spins.
3. Hund's Rule:
Hund's rule states that electrons will occupy empty orbitals of the same energy level individually before pairing up. This principle ensures maximum electron spin alignment and stability.
Electronic Configuration:
The N shell corresponds to the third energy level (n=3) in an atom. The orbitals in the third energy level are 3s, 3p, and 3d.
1. 3s Orbital:
The 3s orbital can accommodate a maximum of two electrons. As per the Aufbau principle, we fill the lower energy 3s orbital before moving to higher energy orbitals. Therefore, the electronic configuration of the 3s orbital in the N shell is 3s2.
2. 3p Orbitals:
The 3p orbitals (3px, 3py, and 3pz) can accommodate a maximum of six electrons (two in each orbital). According to Hund's rule, electrons will occupy empty orbitals individually before pairing up. Therefore, the electronic configuration of the 3p orbitals in the N shell is 3p6.
3. 3d Orbitals:
The 3d orbitals (3dxy, 3dxz, 3dyz, 3dx2-y2, and 3dz2) can accommodate a maximum of ten electrons (two in each orbital). However, the 3d orbitals are in the next higher energy level (n=4) and are not part of the N shell. Therefore, they do not contribute to the electronic configuration of the stable ion with 10 electrons in the N shell.
Final Electronic Configuration:
Based on the above analysis, the electronic configuration of the stable ion with 10 electrons in the N shell is:
3s2 3p6
This configuration represents a stable ion with a completely filled N shell.
To determine the electronic configuration of the stable ion with 10 electrons in the N shell, we need to understand the rules governing electron filling in atomic orbitals.
1. Aufbau Principle:
The Aufbau principle states that electrons fill the lowest energy orbitals first before moving to higher energy orbitals. Therefore, we begin by filling the lower energy orbitals before moving to the higher ones.
2. Pauli Exclusion Principle:
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can accommodate a maximum of two electrons with opposite spins.
3. Hund's Rule:
Hund's rule states that electrons will occupy empty orbitals of the same energy level individually before pairing up. This principle ensures maximum electron spin alignment and stability.
Electronic Configuration:
The N shell corresponds to the third energy level (n=3) in an atom. The orbitals in the third energy level are 3s, 3p, and 3d.
1. 3s Orbital:
The 3s orbital can accommodate a maximum of two electrons. As per the Aufbau principle, we fill the lower energy 3s orbital before moving to higher energy orbitals. Therefore, the electronic configuration of the 3s orbital in the N shell is 3s2.
2. 3p Orbitals:
The 3p orbitals (3px, 3py, and 3pz) can accommodate a maximum of six electrons (two in each orbital). According to Hund's rule, electrons will occupy empty orbitals individually before pairing up. Therefore, the electronic configuration of the 3p orbitals in the N shell is 3p6.
3. 3d Orbitals:
The 3d orbitals (3dxy, 3dxz, 3dyz, 3dx2-y2, and 3dz2) can accommodate a maximum of ten electrons (two in each orbital). However, the 3d orbitals are in the next higher energy level (n=4) and are not part of the N shell. Therefore, they do not contribute to the electronic configuration of the stable ion with 10 electrons in the N shell.
Final Electronic Configuration:
Based on the above analysis, the electronic configuration of the stable ion with 10 electrons in the N shell is:
3s2 3p6
This configuration represents a stable ion with a completely filled N shell.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Question Description
Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.?.
Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.?.
Solutions for Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? defined & explained in the simplest way possible. Besides giving the explanation of
Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.?, a detailed solution for Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? has been provided alongside types of Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? theory, EduRev gives you an
ample number of questions to practice Given that the N shell of an element contains 10 electrons,write the electronic configuration of the stable ion.? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















