BPSC (Bihar) Exam > BPSC (Bihar) Questions > In a dry cell , which of the following are u...
Start Learning for Free
In a dry cell , which of the following are used as a electrolyte ?
- a)Ammonium chloride and zinc chloride
- b)Sodium chloride and calcium chloride
- c)Ammonium chloride and calcium chloride
- d)Magnesium chloride and ammonium chloride
- e)None of the above/More than one of the above
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
In a dry cell , which of the following are used as a electrolyte ?a)A...
Dry cell 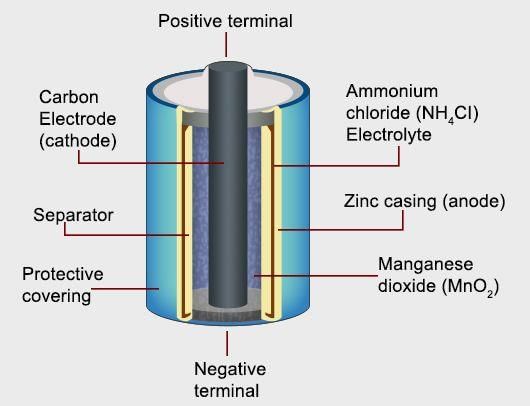
- In the primary batteries, the reaction occurs only once and after use over a period of time battery becomes dead and cannot be reused again.The most familiar example of this type is the dry cell which is used commonly in our transistors and clocks.
- The cell consists of a zinc container that also acts as anode and the cathode is a carbon (graphite) rod surrounded by powdered manganese dioxide and carbon .
- The space between the electrodes is filled by a moist paste of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2 ).
- The electrode reactions are complex, but they can be written approximately as follows :
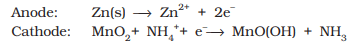
- In the reaction at cathode, manganese is reduced from the + 4 oxidation state to the +3 state. Ammonia produced in the reaction forms a complex with Zn2+ to give [Zn (NH3 ) 4 ] 2+. The cell has a potential of nearly 1.5 V.

Free Test
FREE
| Start Free Test |
Community Answer
In a dry cell , which of the following are used as a electrolyte ?a)A...
Dry cell 
- In the primary batteries, the reaction occurs only once and after use over a period of time battery becomes dead and cannot be reused again.The most familiar example of this type is the dry cell which is used commonly in our transistors and clocks.
- The cell consists of a zinc container that also acts as anode and the cathode is a carbon (graphite) rod surrounded by powdered manganese dioxide and carbon .
- The space between the electrodes is filled by a moist paste of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2 ).
- The electrode reactions are complex, but they can be written approximately as follows :
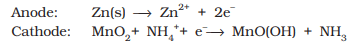
- In the reaction at cathode, manganese is reduced from the + 4 oxidation state to the +3 state. Ammonia produced in the reaction forms a complex with Zn2+ to give [Zn (NH3 ) 4 ] 2+. The cell has a potential of nearly 1.5 V.

Attention BPSC (Bihar) Students!
To make sure you are not studying endlessly, EduRev has designed BPSC (Bihar) study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in BPSC (Bihar).

|
Explore Courses for BPSC (Bihar) exam
|

|
Similar BPSC (Bihar) Doubts
In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer?
Question Description
In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? for BPSC (Bihar) 2024 is part of BPSC (Bihar) preparation. The Question and answers have been prepared according to the BPSC (Bihar) exam syllabus. Information about In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for BPSC (Bihar) 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer?.
In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? for BPSC (Bihar) 2024 is part of BPSC (Bihar) preparation. The Question and answers have been prepared according to the BPSC (Bihar) exam syllabus. Information about In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for BPSC (Bihar) 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer?.
Solutions for In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for BPSC (Bihar).
Download more important topics, notes, lectures and mock test series for BPSC (Bihar) Exam by signing up for free.
Here you can find the meaning of In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In a dry cell , which of the following are used as a electrolyte ?a)Ammonium chloride and zinc chlorideb)Sodium chloride and calcium chloridec)Ammonium chloride and calcium chlorided)Magnesium chloride and ammonium chloridee)None of the above/More than one of the aboveCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice BPSC (Bihar) tests.

|
Explore Courses for BPSC (Bihar) exam
|

|
Suggested Free Tests
Test: Human Environment Interactions the Tropical and The Subtropical Region
Test | 15 questions
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























