MCAT Exam > MCAT Questions > One method for sterilizing the surface of an ...
Start Learning for Free
One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3 or ClF5. What is the molecular geometry of chlorine trifluoride?
- a)trigonal planar
- b)trigonal pyramidal
- c)T-shaped
- d)trigonal bipyramidal
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
One method for sterilizing the surface of an article contaminated with...
Molecular Geometry of Chlorine Trifluoride
Chlorine trifluoride (ClF3) exhibits a unique molecular geometry due to the presence of lone pairs on the chlorine atom.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
- According to VSEPR theory, the shape of a molecule is determined by the repulsion between electron pairs around a central atom.
- Chlorine has 7 valence electrons and forms three single bonds with fluorine atoms, using up 3 of its electrons.
- This leaves 4 electrons, which exist as 2 lone pairs.
Shape Determination
- The presence of these lone pairs affects the geometry:
- The three bonding pairs (Cl-F) arrange themselves to minimize repulsion, forming an equatorial plane.
- The two lone pairs occupy axial positions, leading to a distortion in the shape.
T-Shaped Geometry
- As a result, the molecular geometry of ClF3 can be classified as T-shaped:
- The three fluorine atoms occupy the corners of a “T,” while the lone pairs are positioned above and below the plane.
Comparison with Other Geometries
- This is distinct from:
- Trigonal planar (no lone pairs, 3 bonding pairs)
- Trigonal pyramidal (4 electron pairs, 3 bonding and 1 lone pair)
- Trigonal bipyramidal (5 electron pairs, 5 bonding pairs)
Conclusion
- Therefore, the correct answer for the molecular geometry of chlorine trifluoride is T-shaped, making option 'C' accurate.
Chlorine trifluoride (ClF3) exhibits a unique molecular geometry due to the presence of lone pairs on the chlorine atom.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
- According to VSEPR theory, the shape of a molecule is determined by the repulsion between electron pairs around a central atom.
- Chlorine has 7 valence electrons and forms three single bonds with fluorine atoms, using up 3 of its electrons.
- This leaves 4 electrons, which exist as 2 lone pairs.
Shape Determination
- The presence of these lone pairs affects the geometry:
- The three bonding pairs (Cl-F) arrange themselves to minimize repulsion, forming an equatorial plane.
- The two lone pairs occupy axial positions, leading to a distortion in the shape.
T-Shaped Geometry
- As a result, the molecular geometry of ClF3 can be classified as T-shaped:
- The three fluorine atoms occupy the corners of a “T,” while the lone pairs are positioned above and below the plane.
Comparison with Other Geometries
- This is distinct from:
- Trigonal planar (no lone pairs, 3 bonding pairs)
- Trigonal pyramidal (4 electron pairs, 3 bonding and 1 lone pair)
- Trigonal bipyramidal (5 electron pairs, 5 bonding pairs)
Conclusion
- Therefore, the correct answer for the molecular geometry of chlorine trifluoride is T-shaped, making option 'C' accurate.
Free Test
FREE
| Start Free Test |
Community Answer
One method for sterilizing the surface of an article contaminated with...
Chlorine has seven valence electrons and can expand its octet to form 7 bonds. Chlorine has to be the central atom.
Fluorine has to be single bonded since it cannot expand its octet. Chlorine has 3 single bonds to fluorine, and there are 4 electrons left to make 2 lone pairs.
Count the number of attaching atoms and lone pairs to determine the electronic geometry. For a count of 5, the geometry is trigonal bipyramidal.
The molecular geometries for trigonal bipyramidal electronic geometry are linear, T-shaped, see-saw, and trigonal bipyramidal.
With 3 attached atoms, the molecular geometry is T-shaped. Here is the Lewis dot structure:
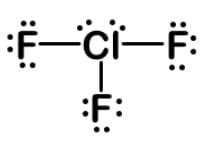

|
Explore Courses for MCAT exam
|

|
Question Description
One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? for MCAT 2025 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for MCAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer?.
One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? for MCAT 2025 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for MCAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer?.
Solutions for One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for MCAT.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
Here you can find the meaning of One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice One method for sterilizing the surface of an article contaminated with bacteria and bacteria spores is using an interhalogen compound such as ClF3or ClF5. What is the molecular geometry of chlorine trifluoride?a)trigonal planarb)trigonal pyramidalc)T-shapedd)trigonal bipyramidalCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice MCAT tests.

|
Explore Courses for MCAT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















