Chemistry Exam > Chemistry Questions > Observe the underlined/bold hydrogen. In HNMR...
Start Learning for Free
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?
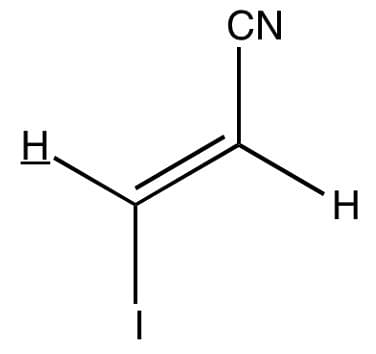

- a)21 lines (multiplet)
- b)7 lines (septet)
- c)1 line (singlet)
- d)2 lines (doublet)
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines...
(1+1)=2 lines
- Within 1H NMR spectroscopy there are a couple important factors to understand, including the ppm shift (delta) and the splitting pattern. Here we are focusing on the splitting pattern for individual hydrogens. This is important for it lays the groundwork for understanding the patterns of peaks seen on large compound NMR’s.
- When determining the splitting of any hydrogen you must use the n+1 rule. Before going into that rule we must understand two things, 1. only nonequivalent hydrogens (protons) couple and 2. usually they only couple to other hydrogens (protons) attached to adjacent carbons. Nonequivalent means the protons occupy their own unique spatial environment with different atoms surrounding them. Typically two hydrogens attached to the same carbon are equivalent (though this isn’t always the case and you must think about where the hydrogens are located in space and see whether they are adjacent to different or similar chemical groups).
- The n+1 rule is performed as follows. N stands for the number of equivalent protons that are not equivalent to the proton of interest (the one we are trying to determine the splitting for). We multiply together each group of protons that are nonequivalent to the proton of interest. For example, lets say there are two groups of protons that are nonequivalent to the proton of interest, in group A there are 2 protons, and in group B there are 3 protons. We would use the n+1 rule for group A and get (2+1) = 3 and for group B get (3+1) = 4. We would then multiply these two numbers together to get the splitting for the proton of interest, thus 3 x 4 = 12 lines that the proton of interest would be split into. Proper use of this rule should allow you to get all NMR splitting questions correct (and elevate your understanding of why a certain NMR printout looks the way it does).

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer?
Question Description
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer?.
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer?.
Solutions for Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer?, a detailed solution for Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Observe the underlined/bold hydrogen. In HNMR, how many spectral lines will that bolded hydrogen be split into?a)21 lines (multiplet)b)7 lines (septet)c)1 line (singlet)d)2 lines (doublet)Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















