Mechanical Engineering Exam > Mechanical Engineering Questions > The heat transferred in a thermodynamic cycle...
Start Learning for Free
The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:
- a)– 8
- b)Zero
- c)10
- d)–10
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
The heat transferred in a thermodynamic cycle of a system consisting o...
Internal energy is a property of a system so 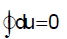
Most Upvoted Answer
The heat transferred in a thermodynamic cycle of a system consisting o...
The net change in internal energy of a system can be calculated using the first law of thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system:
ΔU = Q - W
where ΔU is the change in internal energy, Q is the heat added to the system, and W is the work done by the system.
Given that the system undergoes four processes with heat transfers of 0, 8, 6, and -4 units, respectively, we can calculate the net change in internal energy as follows:
ΔU = Q1 + Q2 + Q3 + Q4 - W
where Q1, Q2, Q3, and Q4 are the heat transfers in each process, and W is the work done by the system.
Since the work done by the system is not given, we cannot calculate the net change in internal energy directly. However, we can use the fact that the net change in internal energy over a complete cycle is zero, since the system returns to its initial state. Therefore, we have:
ΔUcycle = 0 = Q1 + Q2 + Q3 + Q4 - Wcycle
where Wcycle is the net work done by the system over the cycle.
Since Wcycle is not given, we cannot calculate it directly. However, we can use the fact that the net work done by the system over a complete cycle is equal to the area enclosed by the cycle on a PV diagram. Therefore, if we plot the four processes on a PV diagram, we can calculate the area enclosed by the cycle and use it to find the net work done by the system.
Assuming that the system is ideal and undergoes a cyclic process, the area enclosed by the cycle on a PV diagram is a rectangle with sides equal to the initial and final volumes and pressures of the cycle. Therefore, we can calculate the net work done by the system as follows:
Wcycle = (P2 - P1)(V2 - V1)
where P1, V1, P2, and V2 are the initial and final pressures and volumes of the cycle.
Since the pressures and volumes are not given, we cannot calculate the net work done by the system directly. However, we can use the fact that the net work done by the system over a complete cycle is equal to the difference between the heat added to the system and the heat rejected by the system. Therefore, we have:
Wcycle = Q1 + Q2 + Q3 + Q4
Substituting this expression for Wcycle in the equation for ΔUcycle, we get:
ΔUcycle = 0 = Q1 + Q2 + Q3 + Q4 - Q1 - Q2 - Q3 - Q4
Simplifying, we get:
ΔUcycle = 0
Therefore, the net change in internal energy of the system over the complete cycle is zero. Since the system returns to its initial state, the net change in internal energy over any part of the cycle, including the four individual processes, is also zero. Therefore, the correct answer is option B, zero.
ΔU = Q - W
where ΔU is the change in internal energy, Q is the heat added to the system, and W is the work done by the system.
Given that the system undergoes four processes with heat transfers of 0, 8, 6, and -4 units, respectively, we can calculate the net change in internal energy as follows:
ΔU = Q1 + Q2 + Q3 + Q4 - W
where Q1, Q2, Q3, and Q4 are the heat transfers in each process, and W is the work done by the system.
Since the work done by the system is not given, we cannot calculate the net change in internal energy directly. However, we can use the fact that the net change in internal energy over a complete cycle is zero, since the system returns to its initial state. Therefore, we have:
ΔUcycle = 0 = Q1 + Q2 + Q3 + Q4 - Wcycle
where Wcycle is the net work done by the system over the cycle.
Since Wcycle is not given, we cannot calculate it directly. However, we can use the fact that the net work done by the system over a complete cycle is equal to the area enclosed by the cycle on a PV diagram. Therefore, if we plot the four processes on a PV diagram, we can calculate the area enclosed by the cycle and use it to find the net work done by the system.
Assuming that the system is ideal and undergoes a cyclic process, the area enclosed by the cycle on a PV diagram is a rectangle with sides equal to the initial and final volumes and pressures of the cycle. Therefore, we can calculate the net work done by the system as follows:
Wcycle = (P2 - P1)(V2 - V1)
where P1, V1, P2, and V2 are the initial and final pressures and volumes of the cycle.
Since the pressures and volumes are not given, we cannot calculate the net work done by the system directly. However, we can use the fact that the net work done by the system over a complete cycle is equal to the difference between the heat added to the system and the heat rejected by the system. Therefore, we have:
Wcycle = Q1 + Q2 + Q3 + Q4
Substituting this expression for Wcycle in the equation for ΔUcycle, we get:
ΔUcycle = 0 = Q1 + Q2 + Q3 + Q4 - Q1 - Q2 - Q3 - Q4
Simplifying, we get:
ΔUcycle = 0
Therefore, the net change in internal energy of the system over the complete cycle is zero. Since the system returns to its initial state, the net change in internal energy over any part of the cycle, including the four individual processes, is also zero. Therefore, the correct answer is option B, zero.
Free Test
FREE
| Start Free Test |
Community Answer
The heat transferred in a thermodynamic cycle of a system consisting o...
dQ-dW=dU
path-path= point function
So, du=0

|
Explore Courses for Mechanical Engineering exam
|

|
Question Description
The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer?.
The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer?.
Solutions for The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer?, a detailed solution for The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The heat transferred in a thermodynamic cycle of a system consisting of four processes is successively 0, 8, 6 and -4 units. The net change in the internal energy of the system will be:a)– 8b)Zeroc)10d)–10Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















