NEET Exam > NEET Questions > Directions: In the following questions, A sta...
Start Learning for Free
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.
Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.
- a)Both A and R are true and R is the correct explanation of A
- b)Both A and R are true but R is NOT the correct explanation of A
- c)A is true but R is false
- d)Both A and R are False
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Directions: In the following questions, A statement of Assertion (A) i...
Aromatic primary amines cannot be prepared by Gabriel phthalimide synthesis as it is used for the preparation of aliphatic primary amines and not aromatic primary amines.
When phthalimide is treated with aqueous or ethanolic potassium hydroxide, it forms its potassium salt. This salt, when heated with an alkyl halide and then subjected to alkaline hydrolysis, yields the corresponding primary amine.
When phthalimide is treated with aqueous or ethanolic potassium hydroxide, it forms its potassium salt. This salt, when heated with an alkyl halide and then subjected to alkaline hydrolysis, yields the corresponding primary amine.
Aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
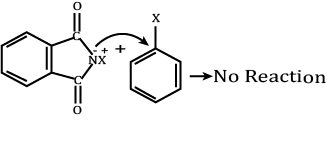
So, the assertion is wrong.
Reason:
Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.
So, the reason is wrong.
Hence, both assertion and reason are wrong.
Most Upvoted Answer
Directions: In the following questions, A statement of Assertion (A) i...
Assertion (A) Analysis
- Aromatic 1° amines can indeed be prepared through the Gabriel Phthalimide synthesis.
- This method involves the reaction of phthalimide with an alkyl halide to form a phthalimide derivative, which can then be hydrolyzed to yield a 1° amine.
- However, the assertion is misleading as it implies a more direct method than is effective for aromatic systems.
Reason (R) Analysis
- The reason states that aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
- This statement is true because aryl halides are quite stable and resistant to nucleophilic attack due to their aromatic nature, which stabilizes the halide bond.
- However, the reason does not directly explain the assertion, as the Gabriel synthesis can be applied to aliphatic amines rather than aromatic amines.
Conclusion
- Since both the assertion and the reason present valid points but do not correlate correctly, the correct answer is option 'D': Both A and R are False.
- The Gabriel Phthalimide synthesis does not yield aromatic amines effectively, and the explanation regarding aryl halides is valid but irrelevant to the process of synthesizing aromatic amines.
In summary, while both statements contain elements of truth, they do not support one another, leading to the conclusion that both are false in the context of the assertion and its reasoning.
- Aromatic 1° amines can indeed be prepared through the Gabriel Phthalimide synthesis.
- This method involves the reaction of phthalimide with an alkyl halide to form a phthalimide derivative, which can then be hydrolyzed to yield a 1° amine.
- However, the assertion is misleading as it implies a more direct method than is effective for aromatic systems.
Reason (R) Analysis
- The reason states that aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
- This statement is true because aryl halides are quite stable and resistant to nucleophilic attack due to their aromatic nature, which stabilizes the halide bond.
- However, the reason does not directly explain the assertion, as the Gabriel synthesis can be applied to aliphatic amines rather than aromatic amines.
Conclusion
- Since both the assertion and the reason present valid points but do not correlate correctly, the correct answer is option 'D': Both A and R are False.
- The Gabriel Phthalimide synthesis does not yield aromatic amines effectively, and the explanation regarding aryl halides is valid but irrelevant to the process of synthesizing aromatic amines.
In summary, while both statements contain elements of truth, they do not support one another, leading to the conclusion that both are false in the context of the assertion and its reasoning.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Question Description
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer?.
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.Assertion (A): Aromatic 1° amines can be prepared by Gabriel Phthalimide synthesis.Reason (R): Aryl halides do not undergo nucleophilic substitution with anion formed by phthalimide.a)Both A and R are true and R is the correct explanation of Ab)Both A and R are true but R is NOT the correct explanation of Ac)A is true but R is falsed)Both A and R are FalseCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















