Chemistry Exam > Chemistry Questions > The [α]D of a 90% optically pure 2-arylp...
Start Learning for Free
The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:
- a)80% and 60°
- b)70% and 40°
- c)80% and 90°
- d)70% and 60°
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The [α]D of a 90% optically pure 2-arylpropanoic acid solutions i...
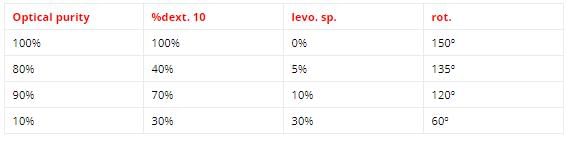
Optically purity also known as enantiomeric excess a compound of 90% B.E. shows 135degree specific rotation.
So, its 100% optically pure isomer will show (135/90) x 100 =150degree specific rot.
At one hour the specific rotation reduced to 120degree.
So, E.E, or optical purity =(120/150) x 100 = 80%
At three hours optical purity is 40%.
So, specific rotation =(150/100) x 40 = 60degree
The over all relation of optical purity, composition and specific rotation can be summarized in the table.
Most Upvoted Answer
The [α]D of a 90% optically pure 2-arylpropanoic acid solutions i...
Explanation:
Optical purity is a measure of the extent to which a sample of a chiral compound contains only one enantiomer. It is denoted by [%] ee or [%] optical purity. The degree of optical purity can be measured by polarimetry, which is a technique that measures the rotation of plane-polarized light as it passes through the sample.
Given data:
- Optical purity = 90%
- []D before treatment = 135
- []D after 1 hour of treatment = 120
- Optical purity after 3 hours of treatment = 40%
Effect of base treatment on optical purity and []D:
When a chiral compound is treated with a base, it undergoes racemization, which means it converts into a racemic mixture containing equal amounts of both enantiomers. This process decreases the optical purity and changes the sign and magnitude of the specific rotation.
1. Optical purity after 1 hour of treatment:
As given, the []D changes from 135 to 120 after 1 hour of treatment. This indicates that the sign of rotation has changed, which means that the enantiomers are no longer present in equal amounts. The change in []D can be calculated using the following formula:
Δ[]D = α×l×c
where,
Δ[]D = change in specific rotation
α = specific rotation (in degrees) at a given wavelength and temperature
l = path length of the sample cell (in dm)
c = concentration of the sample (in g/mL)
Assuming that the path length and concentration remain constant, we can calculate the change in specific rotation as:
Δα = (120 - 135)/(l×c)
Δα = -15/(l×c)
Since the sign of Δα is negative, it means that the enantiomer that was initially present in excess has been consumed more rapidly by the base. Therefore, the optical purity after 1 hour of treatment can be calculated using the following formula:
[%] optical purity = (observed rotation / specific rotation) × 100
where,
observed rotation = measured rotation of the sample after treatment
specific rotation = specific rotation of the pure enantiomer
Let's assume that the pure enantiomer has a specific rotation of +135 (since the initial []D was 135). Then, the observed rotation after 1 hour of treatment can be calculated as:
observed rotation = specific rotation × [%] optical purity / 100
observed rotation = 135 × [%] optical purity / 100
Also, we know that the observed rotation after 1 hour of treatment is 120. Therefore, we can solve for [%] optical purity as follows:
120 = 135 × [%] optical purity / 100
[%] optical purity = 80%
Therefore, the answer is option 'A'.
2. []D after 3 hours of treatment:
As given, the optical purity after 3 hours of treatment is 40%. This means that the mixture contains 60% of the other enantiomer, which has a different specific rotation. Let's assume that the specific rotation of this enantiomer is -100 (just for calculation purposes). Then, the overall specific rotation of the mixture can be calculated as:
overall specific rotation = (specific rotation of enantiomer 1 × [%] optical purity of enantiomer 1 / 100) +
Optical purity is a measure of the extent to which a sample of a chiral compound contains only one enantiomer. It is denoted by [%] ee or [%] optical purity. The degree of optical purity can be measured by polarimetry, which is a technique that measures the rotation of plane-polarized light as it passes through the sample.
Given data:
- Optical purity = 90%
- []D before treatment = 135
- []D after 1 hour of treatment = 120
- Optical purity after 3 hours of treatment = 40%
Effect of base treatment on optical purity and []D:
When a chiral compound is treated with a base, it undergoes racemization, which means it converts into a racemic mixture containing equal amounts of both enantiomers. This process decreases the optical purity and changes the sign and magnitude of the specific rotation.
1. Optical purity after 1 hour of treatment:
As given, the []D changes from 135 to 120 after 1 hour of treatment. This indicates that the sign of rotation has changed, which means that the enantiomers are no longer present in equal amounts. The change in []D can be calculated using the following formula:
Δ[]D = α×l×c
where,
Δ[]D = change in specific rotation
α = specific rotation (in degrees) at a given wavelength and temperature
l = path length of the sample cell (in dm)
c = concentration of the sample (in g/mL)
Assuming that the path length and concentration remain constant, we can calculate the change in specific rotation as:
Δα = (120 - 135)/(l×c)
Δα = -15/(l×c)
Since the sign of Δα is negative, it means that the enantiomer that was initially present in excess has been consumed more rapidly by the base. Therefore, the optical purity after 1 hour of treatment can be calculated using the following formula:
[%] optical purity = (observed rotation / specific rotation) × 100
where,
observed rotation = measured rotation of the sample after treatment
specific rotation = specific rotation of the pure enantiomer
Let's assume that the pure enantiomer has a specific rotation of +135 (since the initial []D was 135). Then, the observed rotation after 1 hour of treatment can be calculated as:
observed rotation = specific rotation × [%] optical purity / 100
observed rotation = 135 × [%] optical purity / 100
Also, we know that the observed rotation after 1 hour of treatment is 120. Therefore, we can solve for [%] optical purity as follows:
120 = 135 × [%] optical purity / 100
[%] optical purity = 80%
Therefore, the answer is option 'A'.
2. []D after 3 hours of treatment:
As given, the optical purity after 3 hours of treatment is 40%. This means that the mixture contains 60% of the other enantiomer, which has a different specific rotation. Let's assume that the specific rotation of this enantiomer is -100 (just for calculation purposes). Then, the overall specific rotation of the mixture can be calculated as:
overall specific rotation = (specific rotation of enantiomer 1 × [%] optical purity of enantiomer 1 / 100) +

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer?
Question Description
The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer?.
The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer?.
Solutions for The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer?, a detailed solution for The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The [α]D of a 90% optically pure 2-arylpropanoic acid solutions is +135°. On treatment with a base at RT for one hour, [α]D changed to +120°. The optical purity is reduced to 40% after 3 hours. If so, the optically purity of the solution after 1 hour, and its [α]D after 3 hours, respectively, would be:a)80% and 60°b)70% and 40°c)80% and 90°d)70% and 60°Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















