Class 12 Exam > Class 12 Questions > One or More than One Options Correct TypeThis...
Start Learning for Free
One or More than One Options Correct Type
This section contains 4 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Select the correct statement(s).
- a)If salt-bridge is removed,potentials falls to zero
- b)Quinhydrone electrode is reversible to H+ ion
- c)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are used
- d)Calomel electrode contains calcium chloride solution in contact with Pt electrode
Correct answer is option 'A,B,C'. Can you explain this answer?
Verified Answer
One or More than One Options Correct TypeThis section contains 4multip...
a) If salt-bridge is removed, anodic and cathodic solution intermix and potential difference falls to zero — correct.
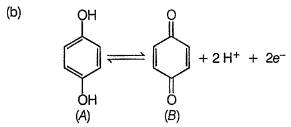
Quinhydrone is a mixture of A and S in (1:1) molar ratio joined by H-bonding. Above equilibrium is set up in aqueous solution.
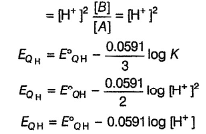
If [H+] changes, EQH also changes. Thus, quinhydrone electrode is reversible to H+ ion correct
(c) Liquid junction potential is minimised by use of concentration cell— correct.
(d) Calomel electrode is Hg(/), Hg2Cl2 (s)| Cl-.
Thus, (d) is incorrect.
Quinhydrone is a mixture of A and S in (1:1) molar ratio joined by H-bonding. Above equilibrium is set up in aqueous solution.
If [H+] changes, EQH also changes. Thus, quinhydrone electrode is reversible to H+ ion correct
(c) Liquid junction potential is minimised by use of concentration cell— correct.
(d) Calomel electrode is Hg(/), Hg2Cl2 (s)| Cl-.
Thus, (d) is incorrect.
Most Upvoted Answer
One or More than One Options Correct TypeThis section contains 4multip...
Answer:
Statement a: If salt-bridge is removed, potentials falls to zero.
Explanation:
When a salt-bridge is present, it helps in maintaining electrical neutrality in the half-cells of an electrochemical cell. It allows the flow of ions between the half-cells, thus completing the circuit and preventing the accumulation of charges. Without a salt-bridge, the accumulation of charges would lead to an increase in the potential difference and an eventual stoppage of the cell reaction. Therefore, if the salt-bridge is removed, the potentials in the cell would fall to zero.
Statement b: Quinhydrone electrode is reversible to H ion.
Explanation:
The quinhydrone electrode is a type of reference electrode that is reversible to the hydrogen ion. It consists of a mixture of quinone and hydroquinone in contact with a hydrogen electrode. The quinone and hydroquinone act as redox couples, and the concentration of hydrogen ions determines the potential of the electrode. The quinhydrone electrode can be used as a reference electrode in pH measurements and is reversible to the hydrogen ion.
Statement c: Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimized if concentration cells are used.
Explanation:
A liquid-junction potential is the potential difference that develops across the boundary of two solutions of different concentration when they come into contact. This potential difference arises due to the difference in the mobility of ions in the two solutions. When concentration cells are used, the two half-cells have the same electrolyte but different concentrations. This minimizes the liquid-junction potential as the difference in ion mobility is eliminated. Therefore, using concentration cells helps to minimize the liquid-junction potential.
Statement d: Calomel electrode contains calcium chloride solution in contact with Pt electrode.
Explanation:
This statement is incorrect. The calomel electrode consists of mercury in contact with a saturated solution of potassium chloride (KCl) and mercurous chloride (Hg2Cl2). It does not contain calcium chloride or a platinum electrode.
In conclusion, the correct statements are a) If salt-bridge is removed, potentials fall to zero, b) Quinhydrone electrode is reversible to H ion, and c) Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimized if concentration cells are used.
Statement a: If salt-bridge is removed, potentials falls to zero.
Explanation:
When a salt-bridge is present, it helps in maintaining electrical neutrality in the half-cells of an electrochemical cell. It allows the flow of ions between the half-cells, thus completing the circuit and preventing the accumulation of charges. Without a salt-bridge, the accumulation of charges would lead to an increase in the potential difference and an eventual stoppage of the cell reaction. Therefore, if the salt-bridge is removed, the potentials in the cell would fall to zero.
Statement b: Quinhydrone electrode is reversible to H ion.
Explanation:
The quinhydrone electrode is a type of reference electrode that is reversible to the hydrogen ion. It consists of a mixture of quinone and hydroquinone in contact with a hydrogen electrode. The quinone and hydroquinone act as redox couples, and the concentration of hydrogen ions determines the potential of the electrode. The quinhydrone electrode can be used as a reference electrode in pH measurements and is reversible to the hydrogen ion.
Statement c: Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimized if concentration cells are used.
Explanation:
A liquid-junction potential is the potential difference that develops across the boundary of two solutions of different concentration when they come into contact. This potential difference arises due to the difference in the mobility of ions in the two solutions. When concentration cells are used, the two half-cells have the same electrolyte but different concentrations. This minimizes the liquid-junction potential as the difference in ion mobility is eliminated. Therefore, using concentration cells helps to minimize the liquid-junction potential.
Statement d: Calomel electrode contains calcium chloride solution in contact with Pt electrode.
Explanation:
This statement is incorrect. The calomel electrode consists of mercury in contact with a saturated solution of potassium chloride (KCl) and mercurous chloride (Hg2Cl2). It does not contain calcium chloride or a platinum electrode.
In conclusion, the correct statements are a) If salt-bridge is removed, potentials fall to zero, b) Quinhydrone electrode is reversible to H ion, and c) Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimized if concentration cells are used.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer?
Question Description
One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer?.
One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer?.
Solutions for One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer?, a detailed solution for One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? has been provided alongside types of One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice One or More than One Options Correct TypeThis section contains 4multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Select the correct statement(s).a)If salt-bridge is removed,potentials falls to zerob)Quinhydrone electrode is reversible to H+ionc)Liquid-junction potential developed across the boundary of the two solutions of different concentration is minimised if concentrations cell are usedd)Calomel electrode contains calcium chloride solution in contact with Pt electrodeCorrect answer is option 'A,B,C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















