Chemistry Exam > Chemistry Questions > Superoxide dismutase contains the metal ions:...
Start Learning for Free
Superoxide dismutase contains the metal ions:
- a)Zn (II) and Ni (II)
- b)Cu (II) and Zn (II)
- c)Ni (II) and Co (III)
- d)Cu (II) and Fe (III)
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu...
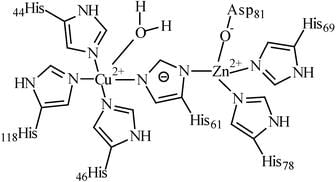
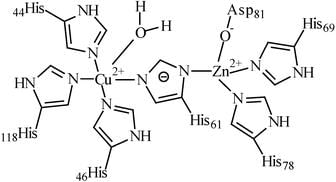
Chemical Structure of Superoxide Dismutase


Free Test
FREE
| Start Free Test |
Community Answer
Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu...
Superoxide dismutase (SOD) is a metalloenzyme that catalyzes the dismutation of superoxide radicals into molecular oxygen and hydrogen peroxide. It contains two metal ions that are essential for its catalytic activity. The metal ions present in SOD are:
Cu (II) and Zn (II)
The metal ions are bound to the protein through a series of histidine residues and are arranged in a specific geometry that is crucial for the enzyme's activity. The Cu (II) ion is located at the active site, where it acts as a Lewis acid to facilitate the oxidation of the superoxide radical. The Zn (II) ion is located at the dimer interface and is involved in stabilizing the protein structure.
The role of Cu (II) and Zn (II) ions in SOD catalysis:
Cu (II) ion:
1. The Cu (II) ion undergoes a redox reaction with the superoxide radical, forming Cu (I) and superoxide anion.
2. The Cu (I) ion is then oxidized back to Cu (II) by another superoxide radical, completing the catalytic cycle.
3. The Cu (II) ion also acts as a Lewis acid, facilitating the oxidation of the superoxide radical.
Zn (II) ion:
1. The Zn (II) ion is involved in stabilizing the protein structure by coordinating with the histidine residues at the dimer interface.
2. The Zn (II) ion also helps to maintain the correct geometry of the active site, which is essential for SOD activity.
In conclusion, SOD contains Cu (II) and Zn (II) ions that are essential for its catalytic activity. The Cu (II) ion is located at the active site and facilitates the oxidation of the superoxide radical, while the Zn (II) ion stabilizes the protein structure and maintains the correct geometry of the active site.
Cu (II) and Zn (II)
The metal ions are bound to the protein through a series of histidine residues and are arranged in a specific geometry that is crucial for the enzyme's activity. The Cu (II) ion is located at the active site, where it acts as a Lewis acid to facilitate the oxidation of the superoxide radical. The Zn (II) ion is located at the dimer interface and is involved in stabilizing the protein structure.
The role of Cu (II) and Zn (II) ions in SOD catalysis:
Cu (II) ion:
1. The Cu (II) ion undergoes a redox reaction with the superoxide radical, forming Cu (I) and superoxide anion.
2. The Cu (I) ion is then oxidized back to Cu (II) by another superoxide radical, completing the catalytic cycle.
3. The Cu (II) ion also acts as a Lewis acid, facilitating the oxidation of the superoxide radical.
Zn (II) ion:
1. The Zn (II) ion is involved in stabilizing the protein structure by coordinating with the histidine residues at the dimer interface.
2. The Zn (II) ion also helps to maintain the correct geometry of the active site, which is essential for SOD activity.
In conclusion, SOD contains Cu (II) and Zn (II) ions that are essential for its catalytic activity. The Cu (II) ion is located at the active site and facilitates the oxidation of the superoxide radical, while the Zn (II) ion stabilizes the protein structure and maintains the correct geometry of the active site.

|
Explore Courses for Chemistry exam
|

|
Question Description
Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer?.
Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer?.
Solutions for Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Superoxide dismutase contains the metal ions:a)Zn (II) and Ni (II)b)Cu (II) and Zn (II)c)Ni (II) and Co (III)d)Cu (II) and Fe (III)Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















