Chemistry Exam > Chemistry Questions > Heat of neutralization of strong acid and wea...
Start Learning for Free
Heat of neutralization of strong acid and weak base is:
- a)57.1 KJ mol–1
- b)13.7 KJ mol–1
- c)Less than 13.7 Kcal mol–1
- d)More than 13.7 Kcal mol–1
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol&#...
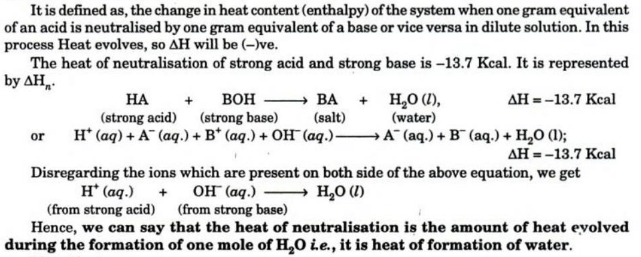
Heat of Neutralization
View all questions of this test

Most Upvoted Answer
Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol&#...
Heat of Neutralization of Strong Acid and Weak Base
When a strong acid and a weak base react together in a neutralization reaction, the heat of neutralization is less than 13.7 kcal/mol. This is because the weak base cannot completely neutralize the strong acid, resulting in the formation of a weakly acidic solution.
Factors Affecting Heat of Neutralization
The heat of neutralization depends on several factors, including:
1. Strength of Acid and Base: Strong acids and bases produce more heat of neutralization compared to weak acids and bases.
2. Concentration of Acid and Base: Higher concentrations of acid and base produce more heat of neutralization.
3. Temperature: The heat of neutralization increases with temperature.
4. Nature of Reaction: The heat of neutralization depends on the nature of the reaction, whether it is an exothermic or endothermic reaction.
Application of Heat of Neutralization
The heat of neutralization is an important concept in various fields, including:
1. Chemical Engineering: Heat of neutralization is used in the design of chemical reactors, which involve neutralization reactions.
2. Pharmaceutical Industry: Heat of neutralization is used in the design of drug formulations, which involve neutralization reactions.
3. Environmental Science: Heat of neutralization is used in the study of acid rain and its effects on the environment.
Conclusion
In conclusion, the heat of neutralization of a strong acid and weak base is less than 13.7 kcal/mol. This is due to the incomplete neutralization of the strong acid by the weak base, resulting in the formation of a weakly acidic solution. The heat of neutralization is an important concept in various fields and is affected by factors such as the strength and concentration of acid and base, temperature, and nature of the reaction.
When a strong acid and a weak base react together in a neutralization reaction, the heat of neutralization is less than 13.7 kcal/mol. This is because the weak base cannot completely neutralize the strong acid, resulting in the formation of a weakly acidic solution.
Factors Affecting Heat of Neutralization
The heat of neutralization depends on several factors, including:
1. Strength of Acid and Base: Strong acids and bases produce more heat of neutralization compared to weak acids and bases.
2. Concentration of Acid and Base: Higher concentrations of acid and base produce more heat of neutralization.
3. Temperature: The heat of neutralization increases with temperature.
4. Nature of Reaction: The heat of neutralization depends on the nature of the reaction, whether it is an exothermic or endothermic reaction.
Application of Heat of Neutralization
The heat of neutralization is an important concept in various fields, including:
1. Chemical Engineering: Heat of neutralization is used in the design of chemical reactors, which involve neutralization reactions.
2. Pharmaceutical Industry: Heat of neutralization is used in the design of drug formulations, which involve neutralization reactions.
3. Environmental Science: Heat of neutralization is used in the study of acid rain and its effects on the environment.
Conclusion
In conclusion, the heat of neutralization of a strong acid and weak base is less than 13.7 kcal/mol. This is due to the incomplete neutralization of the strong acid by the weak base, resulting in the formation of a weakly acidic solution. The heat of neutralization is an important concept in various fields and is affected by factors such as the strength and concentration of acid and base, temperature, and nature of the reaction.
Free Test
FREE
| Start Free Test |
Community Answer
Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol&#...
Weak base in its aqueous form partially ionised therefore some heat of neutralisation used in complete ionisation of weak base hence it's value of heat of neutralisation is less than the 13.7 kcal/mol

|
Explore Courses for Chemistry exam
|

|
Question Description
Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer?.
Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer?.
Solutions for Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Heat of neutralization of strong acid and weak base is:a)57.1 KJ mol–1b)13.7 KJ mol–1c)Less than 13.7 Kcal mol–1 d)More than 13.7 Kcal mol–1Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















