Class 12 Exam > Class 12 Questions > Total vapour pressure of mixture of 1 mol vol...
Start Learning for Free
Total vapour pressure of mixture of 1 mol volatile component A(pºA = 100 mmHg) and 3 mol of volatile component B (pºB = 60 mmHg) is 75 mm. For such case -
- a)There is positive deviation from Raoult's law
- b)Boiling point has been lowered
- c)Force of attraction between A and B is smaller than that between A and A or between B and B
- d)All the above statements are correct
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Total vapour pressure of mixture of 1 mol volatile component A(pº...
As, we know that
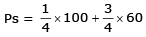
= 25 + 45 mm Hg
= 70 mm Hg
To mm Hg < 75 mm Hg thus there is positive deviation from Raoults law,
so (A) is correct
(B) If v.p. is increasing boiling point will be lowered in that case
(C) is correct that force of attraction between A & B is smaller than that between A and A or between B and B.
So All statement are corrrect
= 25 + 45 mm Hg
= 70 mm Hg
To mm Hg < 75 mm Hg thus there is positive deviation from Raoults law,
so (A) is correct
(B) If v.p. is increasing boiling point will be lowered in that case
(C) is correct that force of attraction between A & B is smaller than that between A and A or between B and B.
So All statement are corrrect
Most Upvoted Answer
Total vapour pressure of mixture of 1 mol volatile component A(pº...
Explanation:
The given mixture contains two volatile components A and B in the ratio 1:3. The total vapour pressure of the mixture is 75 mmHg.
Raoult's Law states that the partial pressure of a component in a solution is directly proportional to its mole fraction in the solution. Mathematically, it can be written as:
PA = XA x P°A
PB = XB x P°B
where PA and PB are the partial pressures of components A and B respectively, XA and XB are their mole fractions, and P°A and P°B are their respective vapour pressures in the pure state.
(a) Positive deviation from Raoult's Law:
The observed total vapour pressure of the mixture is less than the total vapour pressure predicted by Raoult's Law. This indicates that the intermolecular forces between the components A and B are weaker than those between A and A or B and B. As a result, the molecules of A and B are more likely to escape into the vapour phase than they would be if the forces were stronger. This leads to a positive deviation from Raoult's Law.
(b) Boiling point has been lowered:
Since the total vapour pressure of the mixture is less than the sum of the vapour pressures of the pure components, the boiling point of the mixture will be lower than that of the pure components. This is because the molecules of the components escape into the vapour phase more easily than they would if they were alone. Hence, the boiling point has been lowered.
(c) Force of attraction between A and B is smaller than that between A and A or between B and B:
As explained above, the weaker intermolecular forces between A and B lead to a positive deviation from Raoult's Law. This indicates that the force of attraction between A and B is smaller than that between A and A or between B and B.
(d) All the above statements are correct:
Therefore, all the statements (a), (b), and (c) are correct.
The given mixture contains two volatile components A and B in the ratio 1:3. The total vapour pressure of the mixture is 75 mmHg.
Raoult's Law states that the partial pressure of a component in a solution is directly proportional to its mole fraction in the solution. Mathematically, it can be written as:
PA = XA x P°A
PB = XB x P°B
where PA and PB are the partial pressures of components A and B respectively, XA and XB are their mole fractions, and P°A and P°B are their respective vapour pressures in the pure state.
(a) Positive deviation from Raoult's Law:
The observed total vapour pressure of the mixture is less than the total vapour pressure predicted by Raoult's Law. This indicates that the intermolecular forces between the components A and B are weaker than those between A and A or B and B. As a result, the molecules of A and B are more likely to escape into the vapour phase than they would be if the forces were stronger. This leads to a positive deviation from Raoult's Law.
(b) Boiling point has been lowered:
Since the total vapour pressure of the mixture is less than the sum of the vapour pressures of the pure components, the boiling point of the mixture will be lower than that of the pure components. This is because the molecules of the components escape into the vapour phase more easily than they would if they were alone. Hence, the boiling point has been lowered.
(c) Force of attraction between A and B is smaller than that between A and A or between B and B:
As explained above, the weaker intermolecular forces between A and B lead to a positive deviation from Raoult's Law. This indicates that the force of attraction between A and B is smaller than that between A and A or between B and B.
(d) All the above statements are correct:
Therefore, all the statements (a), (b), and (c) are correct.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer?
Question Description
Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer?.
Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Total vapour pressure of mixture of 1 mol volatile component A(pºA= 100 mmHg) and 3 mol of volatile component B (pºB= 60 mmHg) is 75 mm. For such case -a)There is positive deviation from Raoult's lawb)Boiling point has been loweredc)Force of attraction between A and B is smaller than that between A and A or between B and Bd)All the above statements are correctCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















