Chemistry Exam > Chemistry Questions > On dilution, the:a)Specific conductance decre...
Start Learning for Free
On dilution, the:
- a)Specific conductance decreases
- b)Specific conductance increase
- c)Molar conductivity of both weak electrolyte & strong electrolyte increases
- d)Molar conductivity of weak electrolyte becomes more than that of strong electrolyte
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
On dilution, the:a)Specific conductance decreasesb)Specific conductanc...
Weak electrolytes dissociate to a lesser extent and thus, furnish less number of ions for conductance as compared to strong electrolytes. Thus, the conductance of a weak electrolyte is much less than that of a strong electrolyte at same concentration. This is seen in the figure below where acetic acid serves as an example of weak electrolyte.
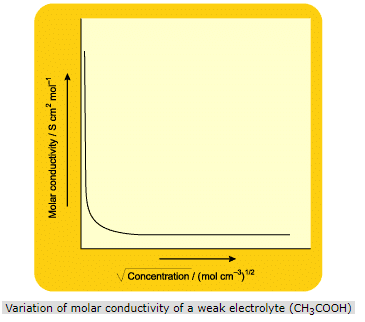
The curve obtained for CH3COOH shows that there is a large increase in the value of molar conductivity with dilution, especially near infinite dilution. This is because as the solution of a weak electrolyte is diluted, its ionization is increased. This results in more number of ions in solution and thus, there is an increase in molar conductivity. However, the conductance of a weak electrolyte never approaches a limiting value.
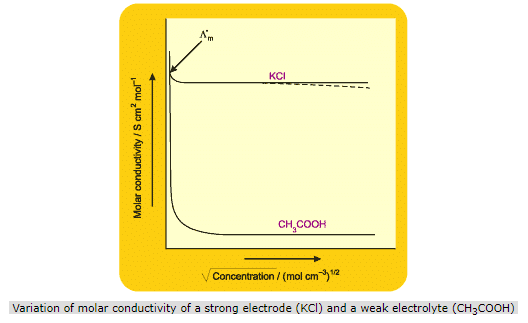
From the plot for a strong electrolyte, it is also seen that the curve for strong electrolyte becomes linear near high dilution (or low concentrations) and thus, can be extrapolated to zero concentration to get the molar conductivity at infinite dilution, i.e. but from the plot for a weak electrolyte, it is seen that the curve near high dilution is almost parallel to the Y-axis and hence, such an extrapolation is not possible for weak electrolytes. Thus, it is not possible to calculate the maximum limiting value of molar conductivity for a weak electrolyte both graphically or experimentally.
This problem was solved by Kohlrausch who formulated a law for calculation of the molar conductivities of weak electrolytes at infinite dilution indirectly. You will study it in the next section.
 This question is part of UPSC exam. View all Chemistry courses
This question is part of UPSC exam. View all Chemistry courses
Most Upvoted Answer
On dilution, the:a)Specific conductance decreasesb)Specific conductanc...
Molar conductivity of strong electrolytes: The curve obtained for strong electrolyte shows that there is only a small increase in conductance with dilution. This is because a strong electrolyte is completely dissociated in solution and so the number of ions remains constant. At higher concentrations, the greater inter-ionic attractions retard the motion of ions and, therefore, the conductance falls with increasing concentrations. With decrease in concentration, or with increase in dilution, the ions are far apart and therefore the interionic attractions decrease due to which the conductance increases with dilution and approaches a maximum limiting value at infinite dilution.
Community Answer
On dilution, the:a)Specific conductance decreasesb)Specific conductanc...
C) Molar conductivity of both weak electrolytes and strong electrolytes increases.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer?
Question Description
On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer?.
On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer?.
Solutions for On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice On dilution, the:a)Specific conductance decreasesb)Specific conductance increasec)Molar conductivity of both weak electrolyte & strong electrolyte increasesd)Molar conductivity of weak electrolyte becomes more than that of strong electrolyteCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















