Class 10 Exam > Class 10 Questions > What is double displacement reaction?
Start Learning for Free
What is double displacement reaction?
Community Answer
What is double displacement reaction?
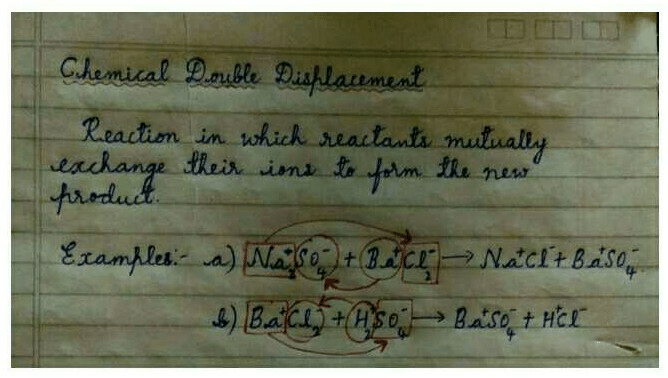
Double displacement reaction is a type of chemical reaction that involves the exchange of ions between two reactants to form new compounds. It is also called metathesis or exchange reaction.
Process of Double Displacement Reaction
In a double displacement reaction, the reactants are usually two ionic compounds or acids. When the reactants are mixed, the positive and negative ions of the two compounds switch places to form two new compounds. The reactants and products are usually in aqueous solution, but they can also be in a solid or gaseous state.
Example of Double Displacement Reaction
An example of a double displacement reaction is the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) to form silver chloride (AgCl) and sodium nitrate (NaNO3):
AgNO3 + NaCl → AgCl + NaNO3
In this reaction, the silver ion (Ag+) from the silver nitrate combines with the chloride ion (Cl-) from the sodium chloride to form silver chloride (AgCl), while the sodium ion (Na+) from the sodium chloride combines with the nitrate ion (NO3-) from the silver nitrate to form sodium nitrate (NaNO3).
Factors Affecting Double Displacement Reaction
The factors that affect double displacement reactions include the nature of the reactants, temperature, concentration, and the presence of a catalyst. Some double displacement reactions are reversible, while others are irreversible.
Applications of Double Displacement Reaction
Double displacement reactions are used in various industrial and laboratory applications, such as in the production of soap, paper, and detergents. They are also used in the purification of metals, the treatment of wastewater, and the synthesis of drugs and chemicals.
Conclusion
In summary, double displacement reaction is a type of chemical reaction that involves the exchange of ions between two reactants to form new compounds. It is widely used in various industrial and laboratory applications and can be affected by various factors.
Process of Double Displacement Reaction
In a double displacement reaction, the reactants are usually two ionic compounds or acids. When the reactants are mixed, the positive and negative ions of the two compounds switch places to form two new compounds. The reactants and products are usually in aqueous solution, but they can also be in a solid or gaseous state.
Example of Double Displacement Reaction
An example of a double displacement reaction is the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl) to form silver chloride (AgCl) and sodium nitrate (NaNO3):
AgNO3 + NaCl → AgCl + NaNO3
In this reaction, the silver ion (Ag+) from the silver nitrate combines with the chloride ion (Cl-) from the sodium chloride to form silver chloride (AgCl), while the sodium ion (Na+) from the sodium chloride combines with the nitrate ion (NO3-) from the silver nitrate to form sodium nitrate (NaNO3).
Factors Affecting Double Displacement Reaction
The factors that affect double displacement reactions include the nature of the reactants, temperature, concentration, and the presence of a catalyst. Some double displacement reactions are reversible, while others are irreversible.
Applications of Double Displacement Reaction
Double displacement reactions are used in various industrial and laboratory applications, such as in the production of soap, paper, and detergents. They are also used in the purification of metals, the treatment of wastewater, and the synthesis of drugs and chemicals.
Conclusion
In summary, double displacement reaction is a type of chemical reaction that involves the exchange of ions between two reactants to form new compounds. It is widely used in various industrial and laboratory applications and can be affected by various factors.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
Question Description
What is double displacement reaction? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about What is double displacement reaction? covers all topics & solutions for Class 10 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is double displacement reaction?.
What is double displacement reaction? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about What is double displacement reaction? covers all topics & solutions for Class 10 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is double displacement reaction?.
Solutions for What is double displacement reaction? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of What is double displacement reaction? defined & explained in the simplest way possible. Besides giving the explanation of
What is double displacement reaction?, a detailed solution for What is double displacement reaction? has been provided alongside types of What is double displacement reaction? theory, EduRev gives you an
ample number of questions to practice What is double displacement reaction? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.