Class 12 Exam > Class 12 Questions > We can obtain picric acid from phenol by:a)Su...
Start Learning for Free
We can obtain picric acid from phenol by:
- a)Sulphonation of phenol
- b)By Reimer Tiemann reaction
- c)Nitration of phenol
- d)Halogenation of phenol
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By ...
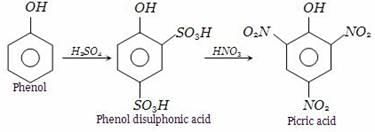
Phenol heated with sulphuric acid gives phenol disulphonic acid, which further on reaction with nitric acid forms picric acid (2,4,6-trinitrophenol).
Free Test
FREE
| Start Free Test |
Community Answer
We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By ...
Obtaining Picric Acid from Phenol
Nitration of Phenol
Picric acid is obtained from phenol by the process of nitration. In this process, phenol is first treated with a mixture of concentrated nitric acid and concentrated sulfuric acid to form 2,4,6-trinitrophenol, which is commonly known as picric acid.
Steps involved in the process of nitration of phenol:
1. Phenol is mixed with a mixture of concentrated nitric acid and concentrated sulfuric acid.
2. The mixture is heated gently to initiate the reaction.
3. Nitration takes place, and 2,4,6-trinitrophenol is formed.
4. The mixture is then cooled and diluted with water.
5. The resulting solution is then filtered to obtain solid picric acid.
Why Nitration of Phenol?
Phenol is a highly reactive compound that can undergo various chemical reactions. One of the most common reactions of phenol is nitration. Nitration of phenol is a highly exothermic reaction that results in the formation of picric acid.
Picric acid has numerous industrial applications, including its use as a dye, a chemical intermediate, and an explosive. It is also used in the manufacturing of medicines and as a laboratory reagent.
Conclusion:
Thus, picric acid can be obtained from phenol by the process of nitration. This process involves the use of concentrated nitric acid and concentrated sulfuric acid to convert phenol into picric acid. Nitration of phenol is a highly exothermic reaction that requires proper handling and safety precautions.
Nitration of Phenol
Picric acid is obtained from phenol by the process of nitration. In this process, phenol is first treated with a mixture of concentrated nitric acid and concentrated sulfuric acid to form 2,4,6-trinitrophenol, which is commonly known as picric acid.
Steps involved in the process of nitration of phenol:
1. Phenol is mixed with a mixture of concentrated nitric acid and concentrated sulfuric acid.
2. The mixture is heated gently to initiate the reaction.
3. Nitration takes place, and 2,4,6-trinitrophenol is formed.
4. The mixture is then cooled and diluted with water.
5. The resulting solution is then filtered to obtain solid picric acid.
Why Nitration of Phenol?
Phenol is a highly reactive compound that can undergo various chemical reactions. One of the most common reactions of phenol is nitration. Nitration of phenol is a highly exothermic reaction that results in the formation of picric acid.
Picric acid has numerous industrial applications, including its use as a dye, a chemical intermediate, and an explosive. It is also used in the manufacturing of medicines and as a laboratory reagent.
Conclusion:
Thus, picric acid can be obtained from phenol by the process of nitration. This process involves the use of concentrated nitric acid and concentrated sulfuric acid to convert phenol into picric acid. Nitration of phenol is a highly exothermic reaction that requires proper handling and safety precautions.

|
Explore Courses for Class 12 exam
|

|
Question Description
We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer?.
We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer?.
Solutions for We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice We can obtain picric acid from phenol by:a)Sulphonation of phenolb)By Reimer Tiemann reactionc)Nitration of phenold)Halogenation of phenolCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























