Class 11 Exam > Class 11 Questions > Lewis dot structure of NO2-?
Start Learning for Free
Lewis dot structure of NO2-?
Community Answer
Lewis dot structure of NO2-?
Lewis Dot Structure of NO2-
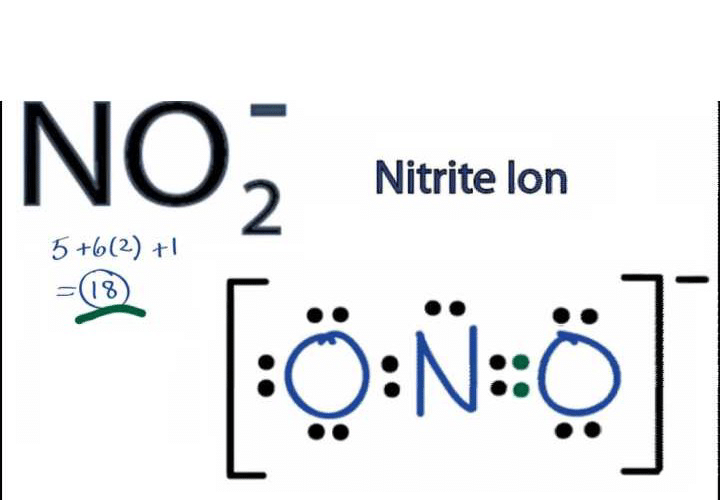
The Lewis dot structure for NO2- (nitrite ion) can be determined by following a set of rules. The Lewis dot structure is a visual representation of the valence electrons in a molecule, showing the bonding and non-bonding electrons.
Rules for drawing Lewis dot structures:
1. Count the total number of valence electrons:
- Nitrogen (N) has 5 valence electrons.
- Oxygen (O) has 6 valence electrons.
- Since there are two oxygen atoms in NO2-, the total number of valence electrons will be 5 + (2 * 6) + 1 (negative charge on the ion) = 18.
2. Determine the central atom:
- In NO2-, nitrogen (N) is the central atom since it is less electronegative than oxygen (O).
3. Connect the atoms with single bonds:
- Place the nitrogen atom in the center and connect it to the two oxygen atoms with single bonds. Each bond consists of a pair of electrons.
4. Distribute the remaining electrons:
- Place the remaining 14 electrons around the oxygen atoms, giving each oxygen atom a lone pair of electrons (two dots) and a single bond with nitrogen.
5. Check the octet rule for each atom:
- The octet rule states that each atom (except hydrogen) should have 8 valence electrons around it. In some cases, elements can have expanded octets, allowing more than 8 electrons.
- In NO2-, nitrogen has 5 valence electrons and is surrounded by 5 more electrons (2 from each oxygen atom and 1 from the negative charge) fulfilling the octet rule.
- Each oxygen atom has 6 valence electrons and is surrounded by 6 more electrons (2 from the nitrogen atom and 4 in the form of lone pairs) fulfilling the octet rule.
Lewis dot structure of NO2-:
N:O:O- (The colon represents a single bond, and the dash represents a lone pair)
.
. .
:
N
. .
:
O-
In the Lewis dot structure of NO2-, the nitrogen atom is in the center, bonded to two oxygen atoms. Each oxygen atom has a lone pair of electrons. The negative charge is placed on one of the oxygen atoms, indicated by the extra electron. The structure satisfies the octet rule for each atom, and the total number of valence electrons is conserved.
The Lewis dot structure for NO2- (nitrite ion) can be determined by following a set of rules. The Lewis dot structure is a visual representation of the valence electrons in a molecule, showing the bonding and non-bonding electrons.
Rules for drawing Lewis dot structures:
1. Count the total number of valence electrons:
- Nitrogen (N) has 5 valence electrons.
- Oxygen (O) has 6 valence electrons.
- Since there are two oxygen atoms in NO2-, the total number of valence electrons will be 5 + (2 * 6) + 1 (negative charge on the ion) = 18.
2. Determine the central atom:
- In NO2-, nitrogen (N) is the central atom since it is less electronegative than oxygen (O).
3. Connect the atoms with single bonds:
- Place the nitrogen atom in the center and connect it to the two oxygen atoms with single bonds. Each bond consists of a pair of electrons.
4. Distribute the remaining electrons:
- Place the remaining 14 electrons around the oxygen atoms, giving each oxygen atom a lone pair of electrons (two dots) and a single bond with nitrogen.
5. Check the octet rule for each atom:
- The octet rule states that each atom (except hydrogen) should have 8 valence electrons around it. In some cases, elements can have expanded octets, allowing more than 8 electrons.
- In NO2-, nitrogen has 5 valence electrons and is surrounded by 5 more electrons (2 from each oxygen atom and 1 from the negative charge) fulfilling the octet rule.
- Each oxygen atom has 6 valence electrons and is surrounded by 6 more electrons (2 from the nitrogen atom and 4 in the form of lone pairs) fulfilling the octet rule.
Lewis dot structure of NO2-:
N:O:O- (The colon represents a single bond, and the dash represents a lone pair)
.
. .
:
N
. .
:
O-
In the Lewis dot structure of NO2-, the nitrogen atom is in the center, bonded to two oxygen atoms. Each oxygen atom has a lone pair of electrons. The negative charge is placed on one of the oxygen atoms, indicated by the extra electron. The structure satisfies the octet rule for each atom, and the total number of valence electrons is conserved.

|
Explore Courses for Class 11 exam
|

|
Question Description
Lewis dot structure of NO2-? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Lewis dot structure of NO2-? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Lewis dot structure of NO2-?.
Lewis dot structure of NO2-? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Lewis dot structure of NO2-? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Lewis dot structure of NO2-?.
Solutions for Lewis dot structure of NO2-? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Lewis dot structure of NO2-? defined & explained in the simplest way possible. Besides giving the explanation of
Lewis dot structure of NO2-?, a detailed solution for Lewis dot structure of NO2-? has been provided alongside types of Lewis dot structure of NO2-? theory, EduRev gives you an
ample number of questions to practice Lewis dot structure of NO2-? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.