JEE Exam > JEE Questions > If an electron is in any higher state n = n a...
Start Learning for Free
If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to 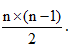
If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to 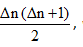
where Δ n = n 2 – n 1 .
Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).
Q.
Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)
- a)2 : 1
- b)1 : 2
- c)1 : 1
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
If an electron is in any higher state n = n and makes a transition to ...
The number of spectral lines will be same no matter how many atoms more than required number of atoms are provided.in 1st sample sum of spectral lines in H and He+ is n1.so n2 will also be equal to n1 as explained earlier.so n1:n2=1:1

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer?
Question Description
If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer?.
If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer?.
Solutions for If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If an electron is in any higher state n = n and makes a transition to ground state, then total no. of different photons emitted is equal to If an electrons is in any higher state n = n2 and makes a transition to another excited state n = n1, then total no. of different photons emitted is equal to where Δ n = n 2 – n 1 .Note: In case of single isolated atom if electron make transition from nth state to the ground state then max. number of spectral lines observed = ( n – 1).Q.Two samples are taken, in 1st sample mole ratio of H to H+ is 1: 2. In IInd sample mole ratio of H to He+ is 2: 1. If n1 is number of spectral lines produced by 1st sample and number of spectral lines produced by lino sample is n2 then n1: n2 will be – (There is sufficient number of both types of atom and both are in same excited state in both sample)a)2 : 1b)1 : 2c)1 : 1d)None of theseCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























