Chemistry Exam > Chemistry Questions > Hybridization and shape of N(SiH3)3 is:a)Sp3,...
Start Learning for Free
Hybridization and shape of N(SiH3)3 is:
- a)Sp3, tetrahedral
- b)sp2, planar
- c)sp3, planar
- d)sp2, angular
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planar...
To determine the structure and hybridization of the molecule N(SiH3)3, we can follow these steps:
Step 1: Identify the central atom and its valence electrons
The central atom in this molecule is nitrogen (N). Nitrogen belongs to group 15 of the periodic table and has 5 valence electrons.
The central atom in this molecule is nitrogen (N). Nitrogen belongs to group 15 of the periodic table and has 5 valence electrons.
Step 2: Determine the bonding and lone pairs
In N(SiH3)3, nitrogen forms three sigma bonds with three silicon (Si) atoms from the three SiH3 groups. Since nitrogen has 5 valence electrons and it uses 3 of them to form bonds, it will have 2 electrons left, which will form a lone pair.
In N(SiH3)3, nitrogen forms three sigma bonds with three silicon (Si) atoms from the three SiH3 groups. Since nitrogen has 5 valence electrons and it uses 3 of them to form bonds, it will have 2 electrons left, which will form a lone pair.
Step 3: Calculate the steric number
The steric number is calculated as the number of sigma bonds plus the number of lone pairs. Here, nitrogen has:
- 3 sigma bonds (to Si)
- 1 lone pair
The steric number is calculated as the number of sigma bonds plus the number of lone pairs. Here, nitrogen has:
- 3 sigma bonds (to Si)
- 1 lone pair
Thus, the steric number is 3+1=4.
Step 4: Determine the hybridization
For a steric number of 4, the hybridization is sp3. However, since there is a lone pair, the molecular geometry will be affected.
For a steric number of 4, the hybridization is sp3. However, since there is a lone pair, the molecular geometry will be affected.
Step 5: Identify the molecular geometry
With one lone pair and three bonding pairs, the molecular geometry will be trigonal pyramidal. This is because the lone pair occupies more space and pushes the bonding pairs down.
With one lone pair and three bonding pairs, the molecular geometry will be trigonal pyramidal. This is because the lone pair occupies more space and pushes the bonding pairs down.
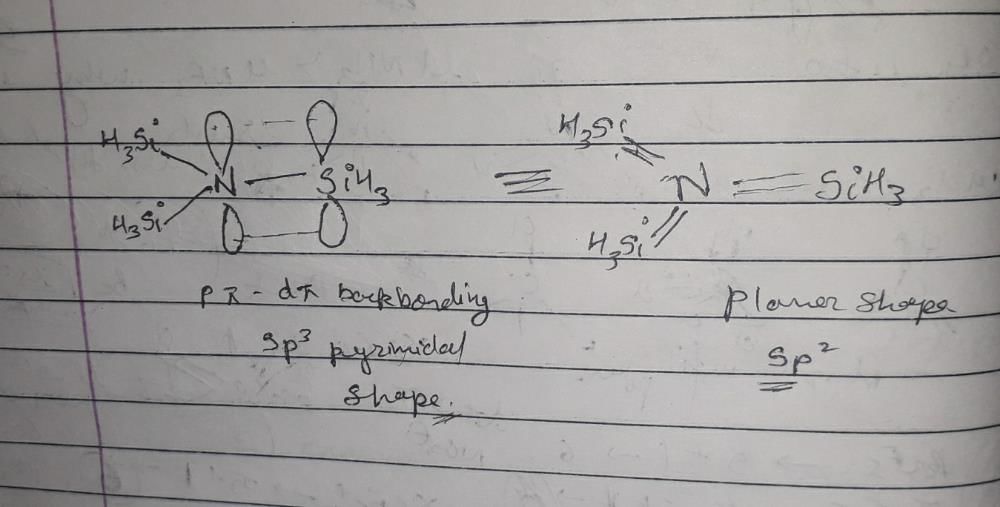
Step 6: Consider back bonding
In N(SiH3)3, there is a possibility of back bonding. Nitrogen can donate its lone pair to the empty d-orbitals of silicon, which can lead to a decrease in hybridization.
In N(SiH3)3, there is a possibility of back bonding. Nitrogen can donate its lone pair to the empty d-orbitals of silicon, which can lead to a decrease in hybridization.
Step 7: Adjust the hybridization due to back bonding
Due to back bonding, the effective hybridization of nitrogen can be considered as sp2 instead of sp3. This means that the nitrogen will have a trigonal planar arrangement around it.
Due to back bonding, the effective hybridization of nitrogen can be considered as sp2 instead of sp3. This means that the nitrogen will have a trigonal planar arrangement around it.
Final Answer
Thus, the structure of N(SiH3)3 is trigonal pyramidal with sp2 hybridization.
Thus, the structure of N(SiH3)3 is trigonal pyramidal with sp2 hybridization.
Most Upvoted Answer
Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planar...
Hybridization and shape of N(SiH3)3:
Hybridization is the process of combining atomic orbitals to form hybrid orbitals that have different properties from the original atomic orbitals. The shape of a molecule is determined by the arrangement of its atoms and the hybridization of its orbitals.
The molecule N(SiH3)3 contains one nitrogen atom and three silicon atoms bonded to it. The nitrogen atom has one unpaired electron in its 2p orbital, which can be used to form three sigma bonds with the silicon atoms. The hybridization of the nitrogen atom is therefore determined by the number of sigma bonds it forms.
Option B is the correct answer because the hybridization of the nitrogen atom in N(SiH3)3 is sp2, and the shape of the molecule is planar.
Explanation:
1. Hybridization of the Nitrogen atom:
The nitrogen atom in N(SiH3)3 forms three sigma bonds with the silicon atoms, which requires three orbitals. Therefore, the nitrogen atom undergoes sp2 hybridization, in which one 2s orbital and two 2p orbitals combine to form three sp2 hybrid orbitals.
2. Shape of the Molecule:
The shape of the molecule is determined by the arrangement of its atoms and the hybridization of its orbitals. In N(SiH3)3, the three sp2 hybrid orbitals on the nitrogen atom are arranged in a trigonal planar geometry, with bond angles of 120 degrees. The three Si-H bonds are also arranged in a trigonal planar geometry around each silicon atom. Therefore, the shape of the molecule is planar.
Conclusion:
In summary, the correct answer for the hybridization and shape of N(SiH3)3 is sp2 and planar, respectively. The nitrogen atom undergoes sp2 hybridization to form three sp2 hybrid orbitals, which are arranged in a trigonal planar geometry around the nitrogen atom. The three silicon atoms are also arranged in a trigonal planar geometry around the nitrogen atom, resulting in a planar shape for the molecule.
Hybridization is the process of combining atomic orbitals to form hybrid orbitals that have different properties from the original atomic orbitals. The shape of a molecule is determined by the arrangement of its atoms and the hybridization of its orbitals.
The molecule N(SiH3)3 contains one nitrogen atom and three silicon atoms bonded to it. The nitrogen atom has one unpaired electron in its 2p orbital, which can be used to form three sigma bonds with the silicon atoms. The hybridization of the nitrogen atom is therefore determined by the number of sigma bonds it forms.
Option B is the correct answer because the hybridization of the nitrogen atom in N(SiH3)3 is sp2, and the shape of the molecule is planar.
Explanation:
1. Hybridization of the Nitrogen atom:
The nitrogen atom in N(SiH3)3 forms three sigma bonds with the silicon atoms, which requires three orbitals. Therefore, the nitrogen atom undergoes sp2 hybridization, in which one 2s orbital and two 2p orbitals combine to form three sp2 hybrid orbitals.
2. Shape of the Molecule:
The shape of the molecule is determined by the arrangement of its atoms and the hybridization of its orbitals. In N(SiH3)3, the three sp2 hybrid orbitals on the nitrogen atom are arranged in a trigonal planar geometry, with bond angles of 120 degrees. The three Si-H bonds are also arranged in a trigonal planar geometry around each silicon atom. Therefore, the shape of the molecule is planar.
Conclusion:
In summary, the correct answer for the hybridization and shape of N(SiH3)3 is sp2 and planar, respectively. The nitrogen atom undergoes sp2 hybridization to form three sp2 hybrid orbitals, which are arranged in a trigonal planar geometry around the nitrogen atom. The three silicon atoms are also arranged in a trigonal planar geometry around the nitrogen atom, resulting in a planar shape for the molecule.
Free Test
FREE
| Start Free Test |
Community Answer
Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planar...

|
Explore Courses for Chemistry exam
|

|
Question Description
Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer?.
Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Hybridization and shape of N(SiH3)3 is:a)Sp3, tetrahedralb)sp2, planarc)sp3, planard)sp2, angularCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























