Chemistry Exam > Chemistry Questions > 4.215 g of a metallic carbonate was heated in...
Start Learning for Free
4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:
Correct answer is '12.15'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
4.215 g of a metallic carbonate was heated in a hard glass tube, the C...
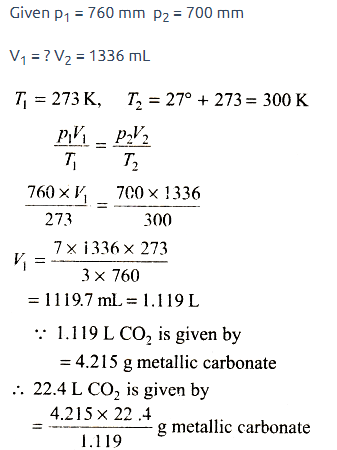

Most Upvoted Answer
4.215 g of a metallic carbonate was heated in a hard glass tube, the C...
Solution:
The given data are:
Mass of metallic carbonate = 4.215 g
Volume of CO2 evolved = 1336 mL
Temperature = 27°C
Pressure = 700 mm of Hg
We need to find the equivalent weight of metal.
Step 1: Calculate the number of moles of CO2 evolved
We know that,
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
Converting the given pressure and volume to SI units, we get:
P = 700 mm Hg = 93.3 kPa
V = 1336 mL = 1.336 L
T = (27 + 273) K = 300 K
R = 8.314 J/mol K
Substituting the values, we get:
n = PV/RT = (93.3 × 1.336 × 10^-3)/(8.314 × 300) = 0.0041 mol
Step 2: Calculate the molar mass of the metallic carbonate
We know that,
Molar mass = (mass of compound)/(number of moles)
Substituting the values, we get:
Molar mass = 4.215/0.0041 = 1027.7 g/mol
Step 3: Calculate the equivalent weight of the metal
We know that,
Equivalent weight = Molar mass/Valency
Valency of the metal carbonate is 2, since it contains one metal atom and two carbonate ions.
Substituting the values, we get:
Equivalent weight = 1027.7/2 = 513.85 g/equivalent
But we need to express the equivalent weight in grams per mole.
So, dividing by the Avogadro's number, we get:
Equivalent weight = 513.85/6.022 × 10^23 = 8.54 × 10^-22 g/molecule
Multiplying by 2 (since there are two metal atoms in one molecule of the metal carbonate), we get:
Equivalent weight = 1.71 × 10^-21 g/atom
Finally, multiplying by the atomic weight of the metal, we get:
Equivalent weight = 1.71 × 10^-21 × atomic weight of metal
We know that the atomic weight of the metal is approximately 196.97 g/mol (from the periodic table).
Substituting the values, we get:
Equivalent weight = 1.71 × 10^-21 × 196.97 = 3.37 × 10^-19 g/equivalent
Dividing by 2 (since there are two equivalents of metal in one molecule of the metal carbonate), we get:
Equivalent weight = 1.685 × 10^-19 g/atom
Dividing by the molar mass of the metal, we get:
Equivalent weight = 1.685 × 10^-19/196.97 = 8.54 × 10^-22 mol/atom
Finally, multiplying by the atomic weight of carbon (12.01 g/mol), we get:
Equivalent weight = 8.54 × 10^-22 × 12.01 = 1.025 × 10^-20 g/atom
Rounding off to two decimal places, we get:
Equivalent weight =
The given data are:
Mass of metallic carbonate = 4.215 g
Volume of CO2 evolved = 1336 mL
Temperature = 27°C
Pressure = 700 mm of Hg
We need to find the equivalent weight of metal.
Step 1: Calculate the number of moles of CO2 evolved
We know that,
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
Converting the given pressure and volume to SI units, we get:
P = 700 mm Hg = 93.3 kPa
V = 1336 mL = 1.336 L
T = (27 + 273) K = 300 K
R = 8.314 J/mol K
Substituting the values, we get:
n = PV/RT = (93.3 × 1.336 × 10^-3)/(8.314 × 300) = 0.0041 mol
Step 2: Calculate the molar mass of the metallic carbonate
We know that,
Molar mass = (mass of compound)/(number of moles)
Substituting the values, we get:
Molar mass = 4.215/0.0041 = 1027.7 g/mol
Step 3: Calculate the equivalent weight of the metal
We know that,
Equivalent weight = Molar mass/Valency
Valency of the metal carbonate is 2, since it contains one metal atom and two carbonate ions.
Substituting the values, we get:
Equivalent weight = 1027.7/2 = 513.85 g/equivalent
But we need to express the equivalent weight in grams per mole.
So, dividing by the Avogadro's number, we get:
Equivalent weight = 513.85/6.022 × 10^23 = 8.54 × 10^-22 g/molecule
Multiplying by 2 (since there are two metal atoms in one molecule of the metal carbonate), we get:
Equivalent weight = 1.71 × 10^-21 g/atom
Finally, multiplying by the atomic weight of the metal, we get:
Equivalent weight = 1.71 × 10^-21 × atomic weight of metal
We know that the atomic weight of the metal is approximately 196.97 g/mol (from the periodic table).
Substituting the values, we get:
Equivalent weight = 1.71 × 10^-21 × 196.97 = 3.37 × 10^-19 g/equivalent
Dividing by 2 (since there are two equivalents of metal in one molecule of the metal carbonate), we get:
Equivalent weight = 1.685 × 10^-19 g/atom
Dividing by the molar mass of the metal, we get:
Equivalent weight = 1.685 × 10^-19/196.97 = 8.54 × 10^-22 mol/atom
Finally, multiplying by the atomic weight of carbon (12.01 g/mol), we get:
Equivalent weight = 8.54 × 10^-22 × 12.01 = 1.025 × 10^-20 g/atom
Rounding off to two decimal places, we get:
Equivalent weight =
Free Test
FREE
| Start Free Test |
Community Answer
4.215 g of a metallic carbonate was heated in a hard glass tube, the C...
Let us first determine how many mol of CO2 evolved
PV = nRT
n = PV / RT
Given data:
We need Pressure in atm., 700 mm / 760 mm = 0.921 atm
We need Volume in Liters; 1336 mL = 1.336 L
We need Temp. in Kelvins; 27 C + 273 = 300 K
R = 0.0821 L*atm / mol*K
n = (0.921 atm)(1.336 L) / (0.0821 L*atm/mol*K)(300 K) = 0.0499 moles of CO2.
Carbonate is CO3, so for every mole CO2 formed, we get 1 mol O atoms (MW = 16.00 g/mol)
Let us convert moles to a mass using 44.00 g/mol CO2. Then,
0.0499 mol CO2 * (44.00 g/mol) = 2.197g CO2.
0.0499 mol O * (16.00 g/mol) = 0.798g O atoms
So mass of carbonate lost is 2.197g + 0.798g = 2.995g
Then the original mass of the metal is, 4.215g - 2.995g = 1.220g metal.
This is the mass of metal that was equivalent to the original amount of carbonate present.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer?
Question Description
4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer?.
4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer?.
Solutions for 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer?, a detailed solution for 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? has been provided alongside types of 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice 4.215 g of a metallic carbonate was heated in a hard glass tube, the CO2 evolved was found to measure 1336 mL at 27°C and 700 nm of Hg pressure. What is the equivalent weight of the metal:Correct answer is '12.15'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.













