Chemistry Exam > Chemistry Questions > Number of unpaired electrons in K2[NiF6] is?C...
Start Learning for Free
Number of unpaired electrons in K2[NiF6] is?
Correct answer is '0'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can...
The atomic number of nickel is 28. Thus, it has 8 electrons in 3d orbital. Here, it is in +2 oxidation state which means its two electrons of its electron from 4s orbital have been removed and the 8 electrons of 3d orbital remain untouched.
Now, F- is a weak field ligand and hence does not pair the electrons in 3d orbital. This means there are 2 unpaired electrons left in 3d orbital. Hence, 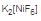 has 2 unpaired electrons.
has 2 unpaired electrons.
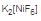 has 2 unpaired electrons.
has 2 unpaired electrons.Most Upvoted Answer
Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can...
Ni is in +4 oxidation state...so there would be rearrangement and back pairing of e
Free Test
FREE
| Start Free Test |
Community Answer
Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can...
Number of unpaired electrons in K2[NiF6]
Explanation:
1. Understanding the structure of K2[NiF6]
K2[NiF6] is the chemical formula for potassium hexafluoroniobate. It consists of potassium cations (K+) and the hexafluoroniobate anion ([NiF6]2-). The [NiF6]2- anion contains a central nickel atom (Ni) surrounded by six fluoride ions (F-).
2. Determining the electron configuration of Ni
To determine the number of unpaired electrons in Ni, we need to know its electron configuration. The atomic number of Ni is 28, so its electron configuration can be written as [Ar] 3d8 4s2. The [Ar] represents the electron configuration of argon (the noble gas before Ni in the periodic table).
3. Analyzing the d-orbitals of Ni
In the electron configuration of Ni, the 3d orbital is partially filled with 8 electrons. The d-orbitals can hold a maximum of 10 electrons. Since there are only 8 electrons in the 3d orbital of Ni, there are two empty d-orbitals available for bonding.
4. Applying Hund's Rule
Hund's rule states that electrons occupy orbitals of the same energy level singly, with the same spin, before pairing up. In other words, electrons prefer to occupy separate orbitals before pairing.
5. Determining the number of unpaired electrons
Since there are two empty d-orbitals available in the 3d orbital of Ni, all the electrons in the 3d orbital will be unpaired. Therefore, the number of unpaired electrons in Ni is 2.
6. Considering the overall compound
In K2[NiF6], there are two potassium cations (K+) and one hexafluoroniobate anion ([NiF6]2-). The overall charge of the compound must be neutral, so the number of positive charges from the potassium cations must balance the negative charge from the anion.
7. Final answer
Since the [NiF6]2- anion has a charge of 2-, and each potassium cation has a charge of +1, it takes two potassium cations to balance the charge of the anion. Therefore, the overall compound has a neutral charge, and the number of unpaired electrons in K2[NiF6] is 0.
Explanation:
1. Understanding the structure of K2[NiF6]
K2[NiF6] is the chemical formula for potassium hexafluoroniobate. It consists of potassium cations (K+) and the hexafluoroniobate anion ([NiF6]2-). The [NiF6]2- anion contains a central nickel atom (Ni) surrounded by six fluoride ions (F-).
2. Determining the electron configuration of Ni
To determine the number of unpaired electrons in Ni, we need to know its electron configuration. The atomic number of Ni is 28, so its electron configuration can be written as [Ar] 3d8 4s2. The [Ar] represents the electron configuration of argon (the noble gas before Ni in the periodic table).
3. Analyzing the d-orbitals of Ni
In the electron configuration of Ni, the 3d orbital is partially filled with 8 electrons. The d-orbitals can hold a maximum of 10 electrons. Since there are only 8 electrons in the 3d orbital of Ni, there are two empty d-orbitals available for bonding.
4. Applying Hund's Rule
Hund's rule states that electrons occupy orbitals of the same energy level singly, with the same spin, before pairing up. In other words, electrons prefer to occupy separate orbitals before pairing.
5. Determining the number of unpaired electrons
Since there are two empty d-orbitals available in the 3d orbital of Ni, all the electrons in the 3d orbital will be unpaired. Therefore, the number of unpaired electrons in Ni is 2.
6. Considering the overall compound
In K2[NiF6], there are two potassium cations (K+) and one hexafluoroniobate anion ([NiF6]2-). The overall charge of the compound must be neutral, so the number of positive charges from the potassium cations must balance the negative charge from the anion.
7. Final answer
Since the [NiF6]2- anion has a charge of 2-, and each potassium cation has a charge of +1, it takes two potassium cations to balance the charge of the anion. Therefore, the overall compound has a neutral charge, and the number of unpaired electrons in K2[NiF6] is 0.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer?
Question Description
Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer?.
Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer?.
Solutions for Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer?, a detailed solution for Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? has been provided alongside types of Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Number of unpaired electrons in K2[NiF6] is?Correct answer is '0'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















