Chemistry Exam > Chemistry Questions > According to the Nernst equation, the potenti...
Start Learning for Free
According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:
- a)60.2 mV
- b)71.0 mV
- c)59.2 mV
- d)None of these.
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
According to the Nernst equation, the potential of an electrode change...
The Nernst equation relates the electrode potential of a half-cell reaction to the concentration of the species involved in the reaction. It can be written as:
E = E° - (RT/nF) * ln(Q)
Where:
E is the electrode potential
E° is the standard electrode potential
R is the gas constant (8.314 J/(mol·K))
T is the temperature in Kelvin
n is the number of electrons transferred in the reaction
F is the Faraday constant (96,485 C/mol)
Q is the reaction quotient, which is the ratio of the concentrations of the products and reactants raised to their stoichiometric coefficients.
In this question, we are given that the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changes by a factor of 10 at 25°C. We need to find the corresponding change in electrode potential at 30°C.
To solve this problem, we need to consider the temperature dependence of the Nernst equation. The temperature term in the Nernst equation is (RT/nF), which represents the effect of temperature on the electrode potential. As the temperature increases, the value of this term increases, resulting in a larger change in the electrode potential.
Since we are given that the change in the ratio of the oxidized and reduced species is the same (a factor of 10) at both temperatures, the only difference is the temperature term in the Nernst equation. We can calculate the change in electrode potential by comparing the temperature terms at 25°C and 30°C.
Let's calculate the temperature term at 25°C:
(25 + 273) K = 298 K
Now, let's calculate the temperature term at 30°C:
(30 + 273) K = 303 K
To find the change in the temperature term, we can subtract the value at 25°C from the value at 30°C:
(303 K) - (298 K) = 5 K
This means that the temperature term at 30°C is 5 K higher than at 25°C.
Since the temperature term is in the denominator of the Nernst equation, a higher temperature term will result in a smaller change in electrode potential. Therefore, the change in electrode potential at 30°C will be smaller than 59.2 mV.
Based on the given options, the correct answer is option C, 60.2 mV, which represents a slightly smaller change in electrode potential compared to 59.2 mV.
E = E° - (RT/nF) * ln(Q)
Where:
E is the electrode potential
E° is the standard electrode potential
R is the gas constant (8.314 J/(mol·K))
T is the temperature in Kelvin
n is the number of electrons transferred in the reaction
F is the Faraday constant (96,485 C/mol)
Q is the reaction quotient, which is the ratio of the concentrations of the products and reactants raised to their stoichiometric coefficients.
In this question, we are given that the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changes by a factor of 10 at 25°C. We need to find the corresponding change in electrode potential at 30°C.
To solve this problem, we need to consider the temperature dependence of the Nernst equation. The temperature term in the Nernst equation is (RT/nF), which represents the effect of temperature on the electrode potential. As the temperature increases, the value of this term increases, resulting in a larger change in the electrode potential.
Since we are given that the change in the ratio of the oxidized and reduced species is the same (a factor of 10) at both temperatures, the only difference is the temperature term in the Nernst equation. We can calculate the change in electrode potential by comparing the temperature terms at 25°C and 30°C.
Let's calculate the temperature term at 25°C:
(25 + 273) K = 298 K
Now, let's calculate the temperature term at 30°C:
(30 + 273) K = 303 K
To find the change in the temperature term, we can subtract the value at 25°C from the value at 30°C:
(303 K) - (298 K) = 5 K
This means that the temperature term at 30°C is 5 K higher than at 25°C.
Since the temperature term is in the denominator of the Nernst equation, a higher temperature term will result in a smaller change in electrode potential. Therefore, the change in electrode potential at 30°C will be smaller than 59.2 mV.
Based on the given options, the correct answer is option C, 60.2 mV, which represents a slightly smaller change in electrode potential compared to 59.2 mV.
Free Test
FREE
| Start Free Test |
Community Answer
According to the Nernst equation, the potential of an electrode change...
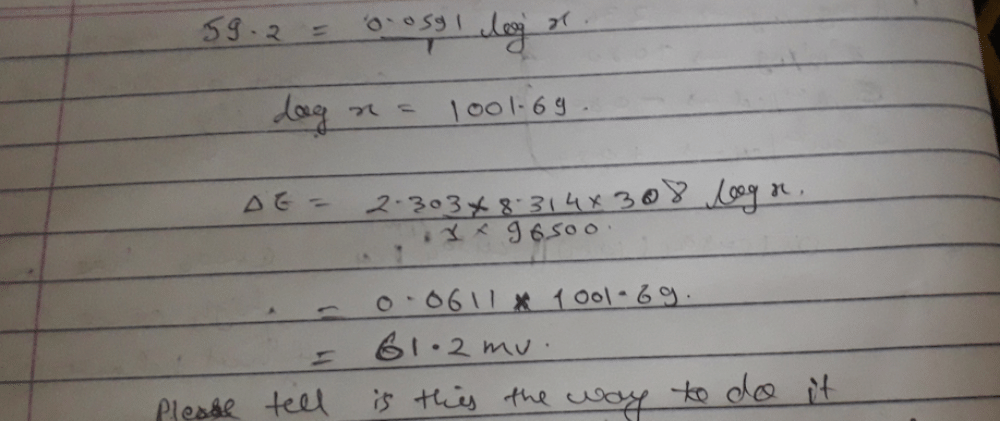

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer?
Question Description
According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer?.
According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer?.
Solutions for According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice According to the Nernst equation, the potential of an electrode changes by 59.2 mV whenever the ratio of the oxidized and the reduced species changed by a factor of 10 at 25°C. What would be the corresponding change in the electrode potential if the experiment is carried out at 30°C:a)60.2 mVb)71.0 mVc)59.2 mVd)None of these.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















