Class 12 Exam > Class 12 Questions > When pyridine is treated with a mixture of ni...
Start Learning for Free
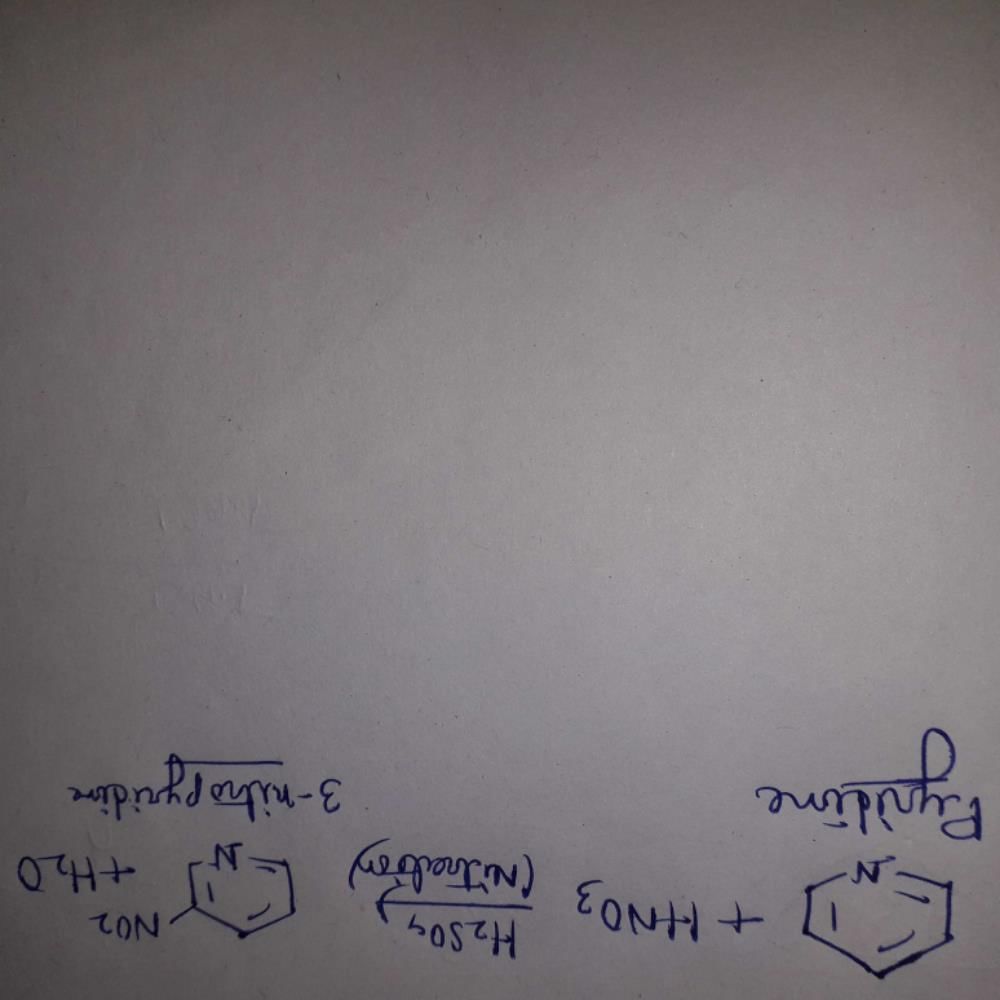
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:
- a)3 – aminopyridine
- b)2 – nitropyridine
- c)3 – nitropyridine
- d)4 – aminopyridine
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
When pyridine is treated with a mixture of nitric and sulfuric acids, ...
In pyridine EAS take splace at 3 position.
Most Upvoted Answer
When pyridine is treated with a mixture of nitric and sulfuric acids, ...
Free Test
FREE
| Start Free Test |
Community Answer
When pyridine is treated with a mixture of nitric and sulfuric acids, ...
Major Product of Pyridine with Nitric and Sulfuric Acids
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product obtained is 3-nitropyridine. This reaction is known as the nitration of pyridine.
Explanation:
The nitration of pyridine involves the substitution of a hydrogen atom on the pyridine ring with a nitro group (-NO2). The reaction is carried out by mixing pyridine with a mixture of nitric and sulfuric acids.
The sulfuric acid acts as a catalyst and also helps in the formation of the nitronium ion (NO2+), which is the electrophile in the reaction. The nitronium ion attacks the pyridine ring and substitutes a hydrogen atom to form a nitro group.
The major product obtained in the reaction is 3-nitropyridine because the nitronium ion prefers to attack the ortho position (position 2) on the pyridine ring due to steric hindrance at the meta position (position 4).
The other possible products are 2-nitropyridine, 4-nitropyridine, and 2,4-dinitropyridine. However, these products are obtained in very small quantities because they are formed by attacking the meta position, which is less favored due to steric hindrance.
Conclusion:
The nitration of pyridine with a mixture of nitric and sulfuric acids results in the formation of 3-nitropyridine as the major product. This reaction is important in the synthesis of various heterocyclic compounds and pharmaceuticals.
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product obtained is 3-nitropyridine. This reaction is known as the nitration of pyridine.
Explanation:
The nitration of pyridine involves the substitution of a hydrogen atom on the pyridine ring with a nitro group (-NO2). The reaction is carried out by mixing pyridine with a mixture of nitric and sulfuric acids.
The sulfuric acid acts as a catalyst and also helps in the formation of the nitronium ion (NO2+), which is the electrophile in the reaction. The nitronium ion attacks the pyridine ring and substitutes a hydrogen atom to form a nitro group.
The major product obtained in the reaction is 3-nitropyridine because the nitronium ion prefers to attack the ortho position (position 2) on the pyridine ring due to steric hindrance at the meta position (position 4).
The other possible products are 2-nitropyridine, 4-nitropyridine, and 2,4-dinitropyridine. However, these products are obtained in very small quantities because they are formed by attacking the meta position, which is less favored due to steric hindrance.
Conclusion:
The nitration of pyridine with a mixture of nitric and sulfuric acids results in the formation of 3-nitropyridine as the major product. This reaction is important in the synthesis of various heterocyclic compounds and pharmaceuticals.

|
Explore Courses for Class 12 exam
|

|
Question Description
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer?.
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer?.
Solutions for When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When pyridine is treated with a mixture of nitric and sulfuric acids, the major product is:a)3 – aminopyridineb)2 – nitropyridinec)3 – nitropyridined)4 – aminopyridineCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























