Chemistry Exam > Chemistry Questions > A C5H12O2 compound has strong infrared absorp...
Start Learning for Free
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:
- a)1, 5-pentanediol
- b)1, 3-dimethoxypropane
- c)2, 2-dimethyl-1, 3-propanediol
- d)2, 4-pentanediol
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1....
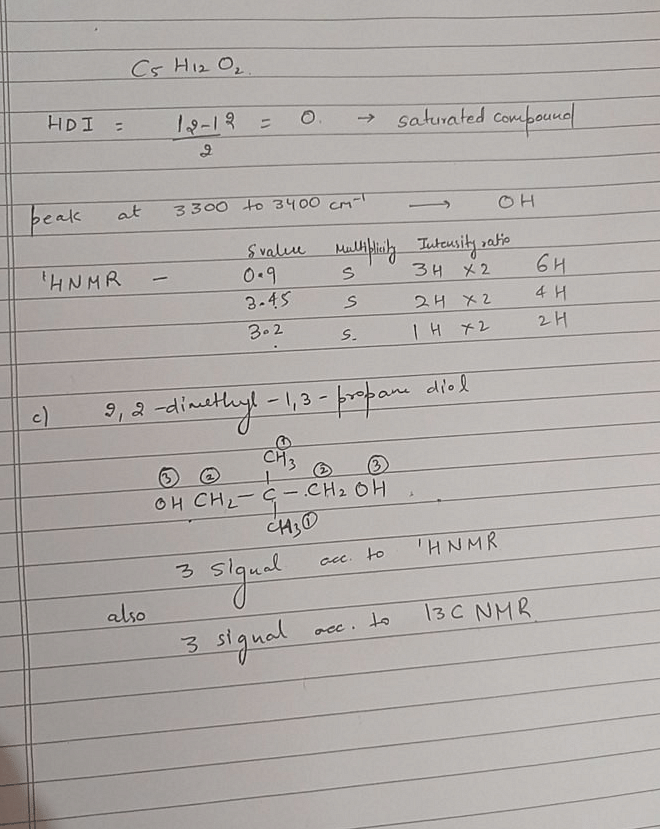
Free Test
FREE
| Start Free Test |
Community Answer
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1....
Δ 1.0, 2.3, and 3.6 ppm. The compound is optically active, and the specific rotation is measured to be +15°.
The infrared absorption at 3300 to 3400 cm-1 suggests the presence of an O-H bond, indicating the presence of an alcohol functional group.
The 1H NMR spectrum shows three singlets at δ 1.0, 2.3, and 3.6 ppm. These singlets indicate the presence of three different types of hydrogens in the compound.
The compound is optically active, as indicated by the specific rotation of +15°. This suggests the presence of a chiral center in the molecule, which results in different enantiomers being formed.
Based on this information, the compound is likely a chiral alcohol with three different types of hydrogens. The structure of the compound cannot be determined solely based on this information, as there are multiple possibilities that fit the given data. Additional information, such as the molecular formula or additional spectroscopic data, would be needed to determine the exact structure of the compound.
The infrared absorption at 3300 to 3400 cm-1 suggests the presence of an O-H bond, indicating the presence of an alcohol functional group.
The 1H NMR spectrum shows three singlets at δ 1.0, 2.3, and 3.6 ppm. These singlets indicate the presence of three different types of hydrogens in the compound.
The compound is optically active, as indicated by the specific rotation of +15°. This suggests the presence of a chiral center in the molecule, which results in different enantiomers being formed.
Based on this information, the compound is likely a chiral alcohol with three different types of hydrogens. The structure of the compound cannot be determined solely based on this information, as there are multiple possibilities that fit the given data. Additional information, such as the molecular formula or additional spectroscopic data, would be needed to determine the exact structure of the compound.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer?
Question Description
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer?.
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer?.
Solutions for A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A C5H12O2 compound has strong infrared absorption at 3300 to 3400cm-1. The 1H NMR spectrum has three singlets at β 0.9, β 3.45 and β 3.2 ppm with relative areas 3:2:1. Addition of D2O to the sample eliminates the lower field signal. The 13C NMR spectrum shows three signals all at higher field than β 100 ppm. Which of the following compounds best fits this data:a)1, 5-pentanediolb)1, 3-dimethoxypropanec)2, 2-dimethyl-1, 3-propanediold)2, 4-pentanediolCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















