Mechanical Engineering Exam > Mechanical Engineering Questions > The triple point on a T-s diagram is ________...
Start Learning for Free
The triple point on a T-s diagram is ________.
- a)a line
- b)a point
- c)a triangle
- d)not present
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
The triple point on a T-s diagram is ________.a)a lineb)a pointc)a tri...
The pressure and temperature at which three phases of a pure substance coexist is called triple point. The triple point is merely the point of intersection of the sublimation and vaporization curves. It has been found that on a ‘p-T’ diagram the triple point is represented by a point and on a ‘p-v’ and T-s diagram it is a line, and on a ‘u-v’ diagram it is a triangle. In the case of ordinary water, the triple point is at a pressure of 4.58 mm Hg and a temperature of 0.01OC.
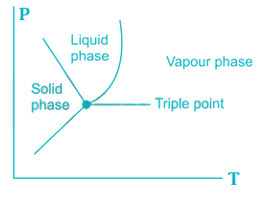
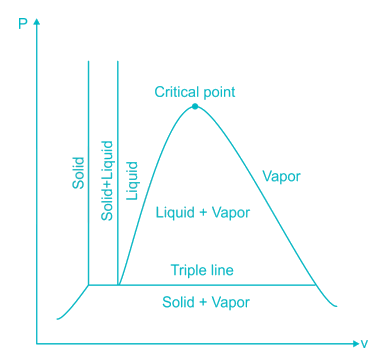
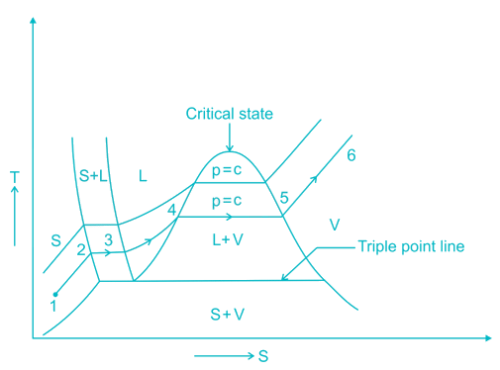
Most Upvoted Answer
The triple point on a T-s diagram is ________.a)a lineb)a pointc)a tri...
Triple Point on a T-s Diagram
The triple point on a T-s (temperature-entropy) diagram is represented by a line, not a point. This is because the triple point is a unique condition where all three phases of a substance (solid, liquid, and gas) coexist in thermodynamic equilibrium. Let's discuss this in more detail.
Definition of Triple Point:
The triple point is a specific combination of temperature and pressure at which a substance can exist in equilibrium in all three phases. At this point, the solid, liquid, and gas phases of the substance can coexist and are in a state of thermodynamic equilibrium.
Representation on a T-s Diagram:
A T-s diagram is a graphical representation of the thermodynamic properties of a substance as it undergoes a change in temperature and entropy. On this diagram, temperature is plotted on the vertical axis (T) and entropy is plotted on the horizontal axis (s).
At the triple point, the substance can exist in all three phases simultaneously. However, the specific values of temperature and entropy at the triple point depend on the substance being considered. For example, the triple point of water occurs at a temperature of 0.01°C and a pressure of 611.657 pascals (Pa).
The line representing the triple point on a T-s diagram is known as the triple line. It separates the regions where two phases coexist from the region where all three phases coexist. The slope of the triple line represents the ratio of the heat of vaporization to the heat of fusion for the substance.
Importance of Triple Point:
The triple point is a crucial reference point in thermodynamics because it provides a fixed and reproducible condition for temperature and pressure. It is often used as a reference in the calibration of temperature scales and pressure measurements.
Conclusion:
In conclusion, the triple point on a T-s diagram is represented by a line, not a point. It is a unique condition where all three phases of a substance coexist in thermodynamic equilibrium. The triple point is important for calibration purposes and is represented by the triple line on a T-s diagram.
The triple point on a T-s (temperature-entropy) diagram is represented by a line, not a point. This is because the triple point is a unique condition where all three phases of a substance (solid, liquid, and gas) coexist in thermodynamic equilibrium. Let's discuss this in more detail.
Definition of Triple Point:
The triple point is a specific combination of temperature and pressure at which a substance can exist in equilibrium in all three phases. At this point, the solid, liquid, and gas phases of the substance can coexist and are in a state of thermodynamic equilibrium.
Representation on a T-s Diagram:
A T-s diagram is a graphical representation of the thermodynamic properties of a substance as it undergoes a change in temperature and entropy. On this diagram, temperature is plotted on the vertical axis (T) and entropy is plotted on the horizontal axis (s).
At the triple point, the substance can exist in all three phases simultaneously. However, the specific values of temperature and entropy at the triple point depend on the substance being considered. For example, the triple point of water occurs at a temperature of 0.01°C and a pressure of 611.657 pascals (Pa).
The line representing the triple point on a T-s diagram is known as the triple line. It separates the regions where two phases coexist from the region where all three phases coexist. The slope of the triple line represents the ratio of the heat of vaporization to the heat of fusion for the substance.
Importance of Triple Point:
The triple point is a crucial reference point in thermodynamics because it provides a fixed and reproducible condition for temperature and pressure. It is often used as a reference in the calibration of temperature scales and pressure measurements.
Conclusion:
In conclusion, the triple point on a T-s diagram is represented by a line, not a point. It is a unique condition where all three phases of a substance coexist in thermodynamic equilibrium. The triple point is important for calibration purposes and is represented by the triple line on a T-s diagram.

|
Explore Courses for Mechanical Engineering exam
|

|
Similar Mechanical Engineering Doubts
The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer?
Question Description
The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer?.
The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer?.
Solutions for The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The triple point on a T-s diagram is ________.a)a lineb)a pointc)a triangled)not presentCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



























