Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Alkalis
Alkalis | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Neutralisation Reactions
- When acids are introduced to water, they generate positively charged hydrogen ions (H+), contributing to the solution's acidity.
- Conversely, when alkalis are added to water, they produce negative hydroxide ions (OH–), rendering the solution alkaline.
- The pH scale serves as a numerical representation indicating the acidity or alkalinity level of a solution, reflecting the concentration of ions within the solution.
- Neutralization reactions occur when acids interact with alkalis.
- During a neutralization reaction, the hydrogen ions (H+) from acids combine with the hydroxide ions (OH–) from alkalis, resulting in the formation of water.
- For instance, when hydrochloric acid undergoes neutralization, sodium chloride and water are generated.
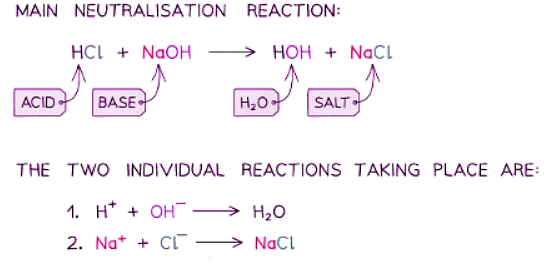
- The simplified ionic equation for acid-alkali neutralizations, which results in a neutral solution due to water's pH of 7, is:
H+ + OH– ⟶ H2O
Hydrogen Ion Concentration & pH
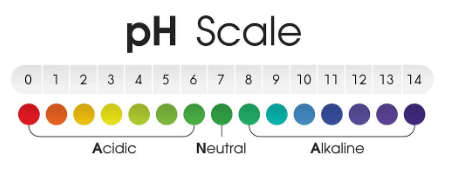
The pH scale
- The pH scale is a tool used to determine the acidity or alkalinity of a solution.
- It ranges from 1 to 14, with values below 7 indicating acidity, above 7 indicating alkalinity, and 7 being neutral
- Acids have pH values below 7, while alkalis have pH values above 7.
- A lower pH signifies higher acidity, while a higher pH indicates higher alkalinity.
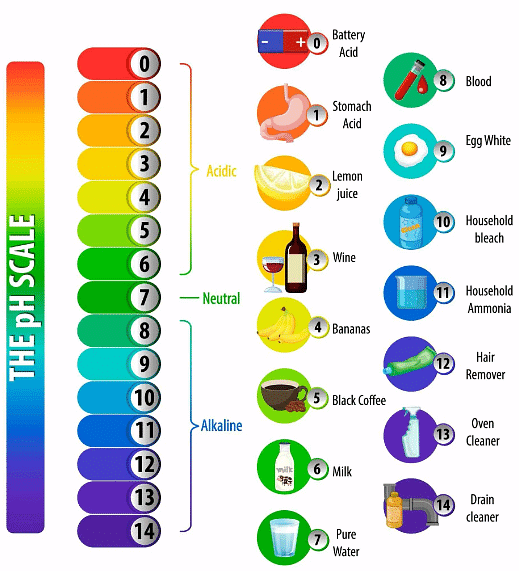
- Lemon juice typically has a pH of around 2, indicating strong acidity.
- Ammonia has a pH of about 11.5, showcasing its alkaline nature.
- The pH scale showing acidity, neutrality, and alkalinity:
- Acids contain hydrogen ions in solution.
- A stronger acid has a higher concentration of hydrogen ions, resulting in a lower pH.
- The concentration of hydroxide ions determines the pH level, with higher concentrations leading to higher pH values.
- pH serves as an indicator of the concentration of H+ ions in a solution, with an inverse relationship between pH and the concentration of H+ ions.
- Universal indicator helps determine pH levels.
- The pH scale is logarithmic, meaning that each change of 1 on the scale represents a change in concentration by a factor of 10
- Therefore an acid with a pH of 3 has ten times the concentration of H+ ions than an acid of pH 4
- An acid with a pH of 2 has 10 x 10 = 100 times the concentration of H+ ions than an acid with a pH of 4
Question for AlkalisTry yourself: Which ions contribute to the acidity of a solution when acids are introduced to water?View Solution
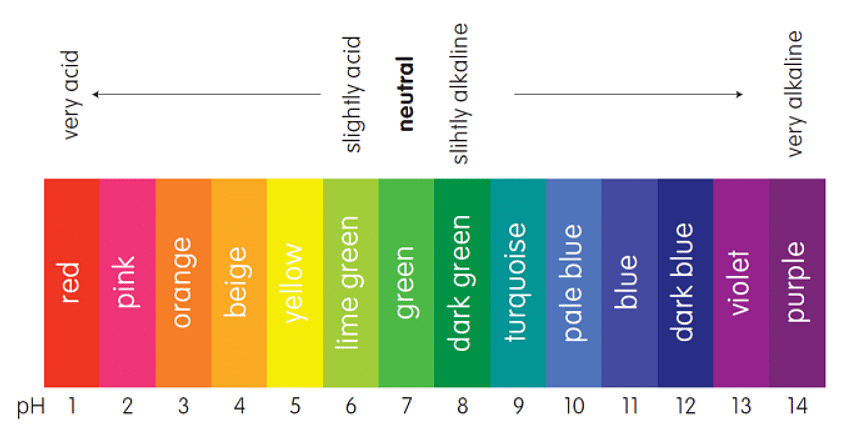
Universal Indicator
- Universal indicator is a blend of different indicators used to measure pH levels.
- Indicators are substances that change color based on the pH of a solution.
- A drop of universal indicator is added to a solution, and its resulting color is compared to a color chart for pH determination.
- The pH scale, along with the Universal Indicator colors, helps determine the pH of a solution by matching colors.
The document Alkalis | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Alkalis - Chemistry for GCSE/IGCSE - Class 10
| 1. What is the pH scale and how does it relate to acids and bases? |  |
Ans. The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral, below 7 being acidic, and above 7 being alkaline. Acids have a pH below 7, while bases have a pH above 7.
| 2. How does Universal Indicator work to determine the pH of a solution? |  |
Ans. Universal Indicator is a mixture of several indicators that change color depending on the pH of a solution. By observing the color change, one can determine the approximate pH of the solution.
| 3. Can you provide examples of common acids and bases found in everyday life? |  |
Ans. Common acids include citric acid in lemons and acetic acid in vinegar, while common bases include sodium hydroxide in drain cleaner and ammonia in window cleaner.
| 4. How do acids and bases react with each other to form salts? |  |
Ans. When an acid reacts with a base, they neutralize each other and form a salt and water. This is known as a neutralization reaction.
| 5. How can the pH of a solution be adjusted using acids and bases? |  |
Ans. By adding an acid to a basic solution, the pH can be lowered, while adding a base to an acidic solution can raise the pH. This process is used in various industries for pH control.
Related Searches





















