Proteins & Amino Acids | General Awareness - Bank Exams PDF Download
| Table of contents |

|
| Amino Acids |

|
| Amino Acid as Dipolar Ions |

|
| Structure of Proteins |

|
| Denaturation of Proteins |

|
Proteins
Proteins are the most abundant biomolecules of the living system. Chief sources of proteins are milk, cheese, pulses, peanuts, fish, meat, etc. They occur in every part of the body and form the fundamental basis of structure and functions of life. They are also required for growth and maintenance of body.
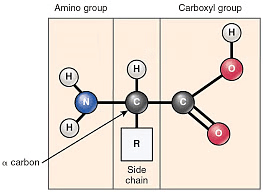 Structure of Protein
Structure of Protein
The word protein is derived from Greek word, “proteios” which means primary or of prime importance. All proteins are polymers of a-amino acids.
Amino Acids
Amino acids are the compounds which contain both an amino group and a carboxy group in their molecules. They constitute a particularly important class of difunctional compounds as they are the building blocks of proteins.
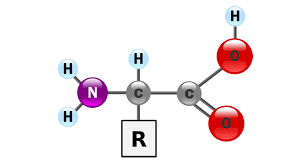 Structure of Amino Acids
Structure of Amino Acids
While several hundred different amino acids are known to occur naturally, 20 of them deserve special mention as they ae preesent in proteins. These amino acids are listed in Table. As given in this table, for amino acids trivial names are common. The convention to use a three letter code, as an abbreviation, for each amino acid is also given in the table. These abbreviations are particularly useful in designating the sequence of amino acids in peptides and proteins which your will study.
Nature of amino acid | E/N.E | Name | Abbreviation | |
Neutral amino acid | NE | Glycine | Gly | |
Neutral amino acid | NE | Alanine | Ala | |
Neutral amino acid | E | Valine | Val | |
Neutral amino acid | E | Leucine | Leu | |
Neutral amino acid | E | Isoleucine | Ile | |
Acidic amino acid | NE | Aspartic Acid | Asp | |
Acidic amino acid | NE | Glutamic Acid | Glu | |
Basic amino acid | E | Lysine | Lys |
Basic amino acid | NE | Arginin | Arg | |
Basic amino acid | NE | Histidine | His | |
Neutral amino acid | E | Methionine | Met | |
Neutral amino acid | NE | Proline | Pro | |
Neutral amino acid | E | Phenylalanine | Phe | |
Neutral amino acid | E | Tryptophan | Trp | |
Neutral amino acid | NE | Serine | Ser | |
Neutral amino acid | E | Threonine | Thr | |
Neutral amino acid | NE | Cysteine | Cys | |
Neutral amino acid | NE | Tyrosine | Tyr |
E = essential amino acid NE = Non essential amino acid
Amino Acid as Dipolar Ions
Amino Acids contain both a basic group (-NH2) and an acidic group (-COOH). In the dry solid state, amino acids exist as dipolar ions, a form in which the carboxyl group is present as a carboxylate ion, -CO2-, and the amino group is present as an aminium ion, -NH3 (Dipolar ions are also called zwitter ions.) In aqueous solution, an equilibrium exists between the dipolar ion and the anionic and cationic forms of amino acids.
If alanine is dissolved in a strongly acidic solution (e.g. pH 0), it is present in mainly a net cationic form. In this state, the amine group is protonated (bears a formal 1 charge) and the carboxylic acid group is neutral (has no formal charge). As is typical of α-amino acids, the pKa for the carboxylic acid hydrogen of alanine is considerably lower (2.3) than the pKa of an ordinary carboxylic acid (e.g., propanoic acid, pKa= 4.89):
The reason for this enhanced acidity of the carboxyl group in an α-amino acid is the inductive effect of the neighbouring aminium cation, which helps to stabilize the carboxylate anion formed when it loses a proton. Loss of proton from the carboxyl group in a cationic α-amino acid leaves the molecule electrically neutral (in the form of a dipolar ion). This equilibrium is shown in the red-shaded portion of the equation below.
The protonated amine group of an α-amino acid is also acidic, but less so that the carboxylic acid group. The pKa of the animium group in alanine is 9.7. The equilibrium for loss of an aminium proton is shown in the blue-shaded portion of the equation below. The carboxylic acid proton is always lost before a proton from the aminium group in an α-amino acid.
The state of an a-amino acid at any given pH is governed by a combination of two equilibrium, as shown in the above equation for alanine. The isoelectric point (pI) of an amino acid such as alanine is the average of pKa1 and pKa2;
pI = ½ (2.3 9.7) = 6.0 (isoelectric point of alanine)
When a base is added to a solution of the net cationic form of alanine (initially at pH 0, for example), the first proton removed is the carboxylic acid proton, as we have said. In the case of alanine, when a pH of 2.3 is reached, the acid proton will have been removed from half of the molecules. This pH represents the pKa of the alanine carboxylic acid proton, as can be demonstrated using the Henderson-Hasselbalch equation. The Henderson - Hasselbalch equation shows that for an acid (HA) and its conjugate base (A-),
pKa = pH+ log[HA]/[A-]
Structure of Proteins
Proteins consist of chains of α-amino acids linked together by peptide bonds. Chemically, a peptide bond forms between the -COOH group of one amino acid and the -NH2 group of another, resulting in the release of a water molecule.
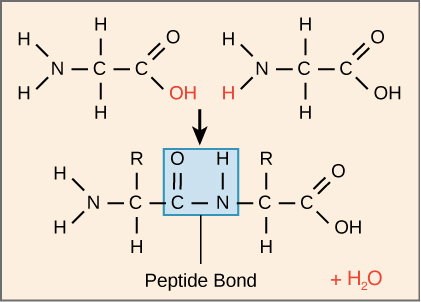
This reaction yields a dipeptide, composed of two amino acids, such as glycylalanine formed from glycine and alanine. Additional amino acids can join this chain, creating tripeptides, tetrapeptides, and so forth, with the resulting molecules termed tripeptides, tetrapeptides, and so on.
When the number of amino acids exceeds ten, the chain is typically referred to as a polypeptide, and if it exceeds a hundred, with a molecular mass surpassing 10,000u, it's generally classified as a protein. However, the boundary between polypeptides and proteins isn't always clear-cut, with smaller polypeptides sometimes considered proteins if they exhibit a defined protein-like structure, as seen in insulin, which contains 51 amino acids.
Proteins can be classified into two types on the basis of their molecular shape.
- Fibrous proteins- When the polypeptide chains run parallel and are held together by hydrogen and disulphide bonds, then fibre– like structure is formed. Such proteins are generally insoluble in water. Some common examples are keratin (present in hair, wool, silk) and myosin (present in muscles), etc.
- Globular proteins- This structure results when the chains of polypeptides coil around to give a spherical shape. These are usually soluble in water. Insulin and albumins are the common examples of globular proteins. Structure and shape of proteins can be studied at four different levels, i.e., primary, secondary, tertiary and quaternary, each level being more complex than the previous one.
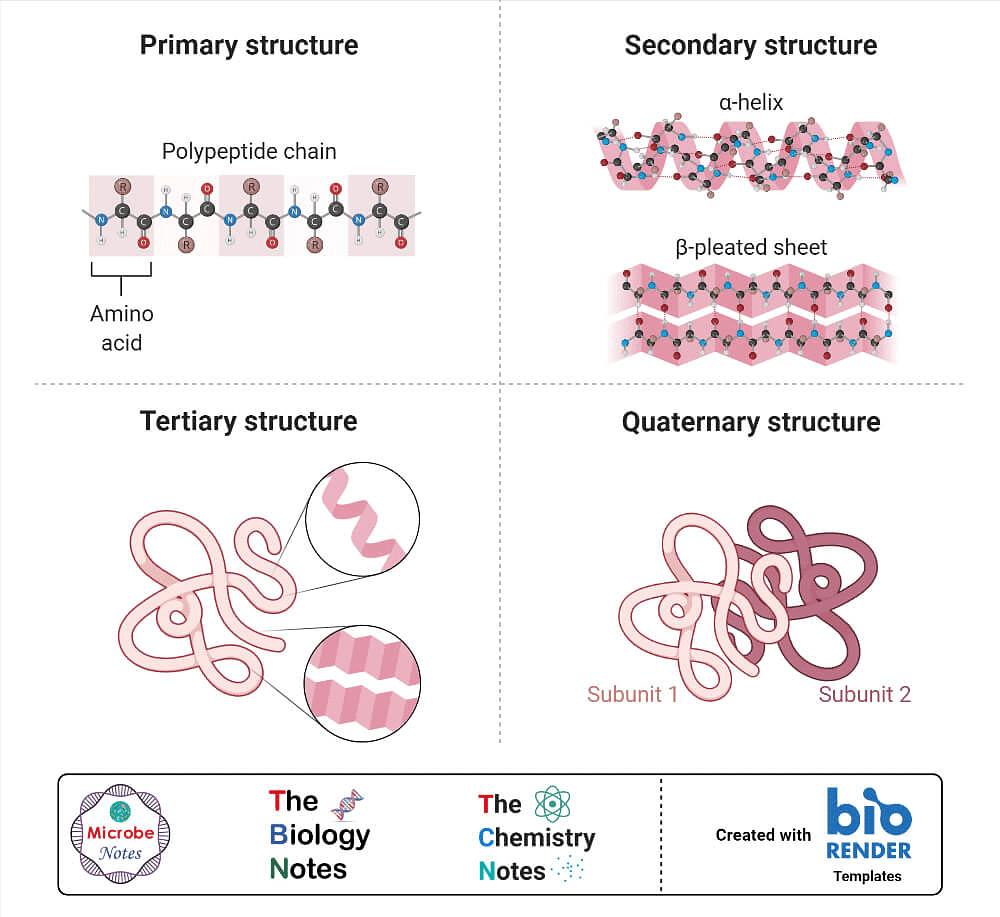
(i) Primary structure of proteins: Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is said to be the primary structure of that protein. Any change in this primary structure i.e., the sequence of amino acids creates a different protein.
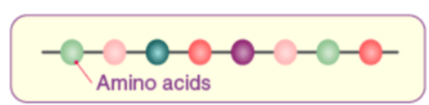
(ii) Secondary structure of proteins: The secondary structure of protein refers to the shape in which a long polypeptide chain can exist. They are found to exist in two different types of structures viz. a-helix and b-pleated sheet structure. These structures arise due to the regular folding of the backbone of the polypeptide chain due to hydrogen bonding between and –NH– groups of the peptide bond.
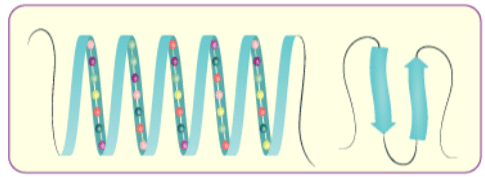
a-Helix is one of the most common ways in which a polypeptide chain forms all possible hydrogen bonds by twisting into a right handed screw (helix) with the –NH group of each amino acid residue hydrogen bonded to the C O of an adjacent turn of the helix . In b-pleated sheet structure all peptide chains are stretched out to nearly maximum extension and then laid side by side which are held together by intermolecular hydrogen bonds. The structure resembles the pleated folds of drapery and therefore is known as b-pleated sheet.
(iii) Tertiary structure of proteins: The tertiary structure of proteins represents overall folding of the polypeptide chains i.e., further folding of the secondary structure. It gives rise to two major molecular shapes viz. fibrous and globular. The main forces which stabilise the 2° and 3° structures of proteins are hydrogen bonds, disulphide linkages, van der Waals and electrostatic forces of attraction.
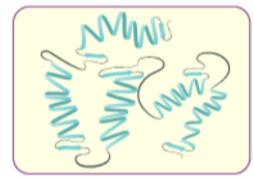
(iv) Quaternary structure of proteins: Some of the proteins are composed of two or more polypeptide chains referred to as sub-units. The spatial arrangement of these subunits with respect to each other is known as quaternary structure.
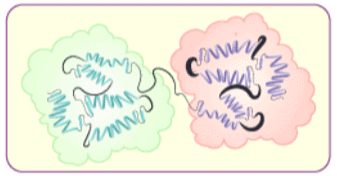
Denaturation of Proteins
- Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein.
- When a protein in its native form, is subjected to physical change like change in temperature or chemical change like change in pH, the hydrogen bonds are disturbed.
- Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
- During denaturation secondary and tertiary structures are destroyed but primary structure remains intact. The coagulation of egg white on boiling is a common example of denaturation. Another example is curdling of milk which is caused due to the formation of lactic acid by the bacteria present in milk.
b) Co-polymers are another type of polymers. These contain more than one sub-unit (or monomer).
Example:
In the above example, styrene and maleic anhydride monomers laternate. Co-polymer can be a block co-polymer.
Example:
Co-polymers can be random as well.
— B - A - A - B - A - B - B - A - B - A -B - B - A —
A and B are monomers.
6. There are many polymers in nature.
Example: Cellulose, starch, pepsin, insulin, egg albumin, rubber, DNA (Deoxyribonucleic acid) etc. These are called Biopolymers.
Man made polymers are, Nylon, Terylene, Polythene, Polystyrene, PVC (Polyvinyl chloride), Bakelite, Perspex, Polysiloxane etc.
7. The properties of a polymer solution are strikingly different from those of a true solution. For example, when polyvinyl alcohol is added to water, it swells.
a) Its shape gets distorted and after a long time it dissolves.
b) When more of polymer is added to a given solvent, saturation point is not reached. The mixture of polymer and solvent assumes a soft dough-like consistency.
8. Addition polymers and condensation polymers are two important types of polymers.
9. Polymer can be described as linear, branched and network.
|
365 videos|700 docs|149 tests
|
FAQs on Proteins & Amino Acids - General Awareness - Bank Exams
| 1. What are amino acids and how are they important in the structure of proteins? |  |
| 2. How do amino acids exist as dipolar ions and why is this important in protein structure? |  |
| 3. What is the significance of the denaturation of proteins and how does it affect their function? |  |
| 4. How are proteins related to amino acids in terms of their biological functions? |  |
| 5. Can the structure of proteins be altered by external factors, and how does this impact their function? |  |















