Baeyer-Villiger Oxidation & Rearrangement | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Definition: What is Baeyer-Villiger Oxidation? |

|
| Examples of Baeyer-Villiger Oxidation |

|
| Mechanism of Baeyer-Villiger Oxidation |

|
| Baeyer-Villiger Rearrangement |

|
Definition: What is Baeyer-Villiger Oxidation?
The Baeyer-Villiger oxidation is the oxidative cleavage of a carbon-carbon bond that is adjacent to a carbonyl functional group. This reaction is used to convert ketones to esters and cyclic ketones to lactones. It can be carried out with peroxy acids (also called peracid) like MCPBA, or with hydrogen peroxide and a Lewis acid. This reaction can be viewed as the insertion of O into one of the C-C bonds adjacent to the carbonyl functional group. For non-symmetrical cyclic ketones, the more highly substituted alkyl group migrates and becomes attached to the inserted O atom.

The history of this reaction goes back to 1899 when German chemists Adolf von Baeyer and Victor Villiger reported it.
Examples of Baeyer-Villiger Oxidation
Baeyer-Villiger oxidation is used in the synthesis of caprolactone from cyclohexanone.
Mechanism of Baeyer-Villiger Oxidation
In this reaction, the ketone is oxidized, whereas the peroxy acid is reduced.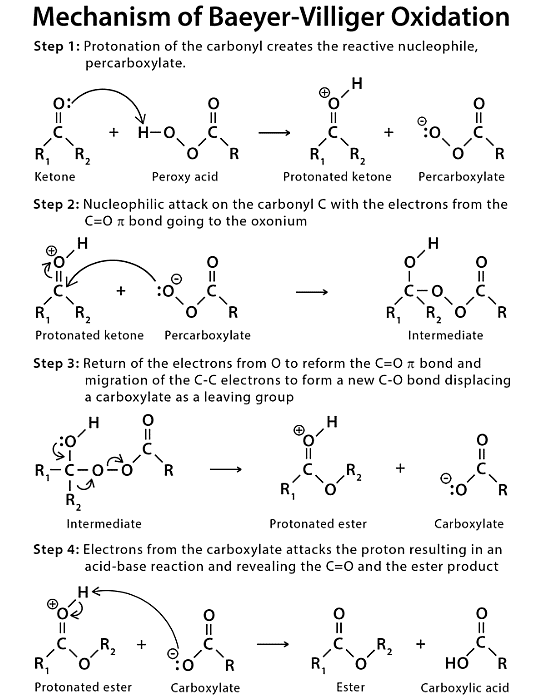
Application of Baeyer-Villiger Oxidation
Baeyer-Villiger oxidation is used in the synthesis of lactones, which contribute significantly to the flavor of fruits, and unfermented and fermented dairy products. Hence, they are used for flavor and fragrance. Polycaprolactone is a biodegradable polyester from which polyurethane is made.
Baeyer-Villiger Rearrangement
The Baeyer-Villiger rearrangement is the conversion of a ketone to an ester via the insertion of an oxygen atom next to the carbonyl.
The reaction involves initial addition of a peroxide to the carbonyl carbon. The resulting adduct undergoes rearrangement to form the ester.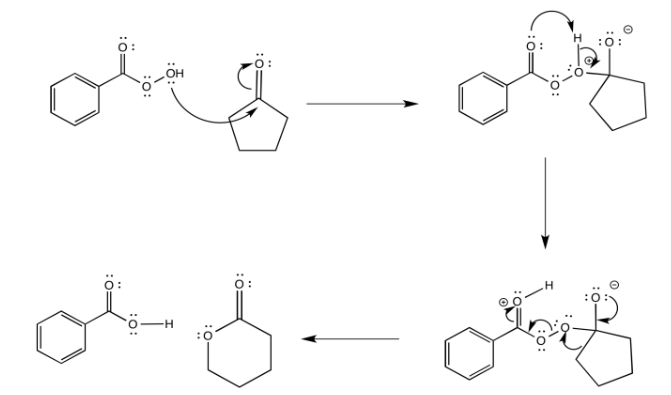
Solved Examples
Example: Predict the products of the following Baeyer-Villiger reactions. Ans:
Ans:
FAQs on Baeyer-Villiger Oxidation & Rearrangement - Chemistry Optional Notes for UPSC
| 1. What is Baeyer-Villiger Oxidation? |  |
| 2. Can you provide examples of Baeyer-Villiger Oxidation? |  |
| 3. What is the mechanism of Baeyer-Villiger Oxidation? |  |
| 4. What is Baeyer-Villiger rearrangement? |  |
| 5. How is Baeyer-Villiger Oxidation relevant to the UPSC exam? |  |

|
Explore Courses for UPSC exam
|

|
















