Benzyne | Chemistry Optional Notes for UPSC PDF Download
Elimination-Addition Mechanism of Nucleophilic Aromatic Substitution. Arynes
The reactivities of aryl halides, such as the halobenzenes, are exceedingly low toward nucleophilic reagents that normally effect substitution with alkyl halides and activated aryl halides. Substitutions do occur under forcing conditions of either high temperatures or very strong bases. For example, chlorobenzene reacts with sodium hydroxide solution at temperatures around 340o and this reaction was once an important commercial process for the production of benzenol (phenol): In addition, aryl chlorides, bromides, and iodides can be converted to areneamines ArNH2 by the conjugate bases of amines. In fact, the reaction of potassium amide with bromobenzene is extremely rapid, even at temperatures as low as −33o with liquid ammonia as solvent:
In addition, aryl chlorides, bromides, and iodides can be converted to areneamines ArNH2 by the conjugate bases of amines. In fact, the reaction of potassium amide with bromobenzene is extremely rapid, even at temperatures as low as −33o with liquid ammonia as solvent:
However, substitution reactions of this type differ from the previously discussed substitutions of activated aryl halides in that rearrangement often occurs. That is, the entering group does not always occupy the same position on the ring as that vacated by the halogen substituent. For example, the hydrolysis of 4-chloromethylbenzene at 340o gives an equimolar mixture of 3- and 4-methylbenzenols:
Even more striking is the exclusive formation of 3-methoxybenzenamine in the amination of 2-chloromethoxybenzene. Notice that this result is a violation of the principle of least structural change:
 The mechanism of this type of reaction has been studied extensively, and much evidence has accumulated in support of a stepwise process, which proceeds first by base-catalyzed elimination of hydrogen halide (HX) from the aryl halide - as illustrated below for the amination of bromobenzene:
The mechanism of this type of reaction has been studied extensively, and much evidence has accumulated in support of a stepwise process, which proceeds first by base-catalyzed elimination of hydrogen halide (HX) from the aryl halide - as illustrated below for the amination of bromobenzene:
Elimination:
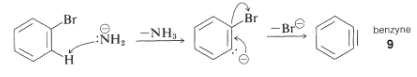
The product of the elimination reaction is a highly reactive intermediate 9 called benzyne, or dehydrobenzene, which differs from benzene in having two less hydrogen and an extra bond between two ortho carbons. Benzyne reacts rapidly with any available nucleophile, in this case the solvent, ammonia, to give an addition product:
Addition
The rearrangements in these reactions result from the attack of the nucleophile at one or the other of the carbons of the extra bond in the intermediate. With benzyne the symmetry is such that no rearrangement would be detected. With substituted benzynes isomeric products may result. Thus 4-methylbenzyne, 10 , from the reaction of hydroxide ion with 4-chloro-1-methylbenzene gives both 3- and 4-methylbenzenols:
In the foregoing benzyne reactions the base that produces the benzyne in the elimination step is derived from the nucleophile that adds in the addition step. This need not always be so, depending on the reaction conditions. In fact, the synthetic utility of aryne reactions depends in large part of the success with which the aryne can be generated by one reagent but captured by another. One such method will be discussed in Section 14-10C and involves organometallic compounds derived from aryl halides. Another method is to generate the aryne by thermal decomposition of a 1,2-disubstituted arene compound such as 11 , in which both substituents are leaving groups - one leaving with an electron pair, the other leaving without:
When 11 decomposes in the presence of an added nucleophile, the benzyne intermediate is trapped by the nucleophile as it is formed. Or, if a conjugated diene is present, benzyne will react with it by a [4 + 2] cycloaddition. In the absence of other compounds with which it can react, benzyne will undergo [2 + 2] cycloaddition to itself:
Solved Examples
Example: When p-chlorotoluene is reacted with NaOH, two products are seen. While when m-chlorotoluene is reacted with NaOH, three products are seen. Explain this.
Ans: When p-chlorotoluene is reacted with NaOH, two products are seen. While when m-chlorotoluene is reacted with NaOH, three products are seen. Explain this.
FAQs on Benzyne - Chemistry Optional Notes for UPSC
| 1. What is the elimination-addition mechanism of nucleophilic aromatic substitution? |  |
| 2. What is an aryne or benzyne intermediate? |  |
| 3. What are the key features of nucleophilic aromatic substitution reactions? |  |
| 4. What are some examples of nucleophilic aromatic substitution reactions involving arynes? |  |
| 5. How do nucleophilic aromatic substitution reactions involving arynes differ from traditional nucleophilic aromatic substitutions? |  |

|
Explore Courses for UPSC exam
|

|

















