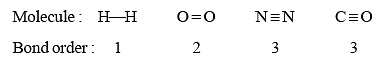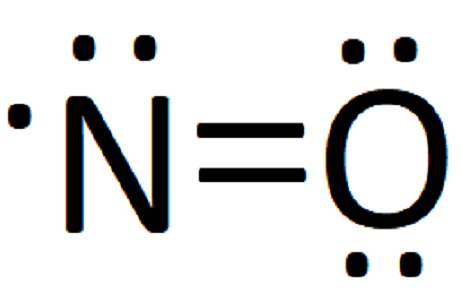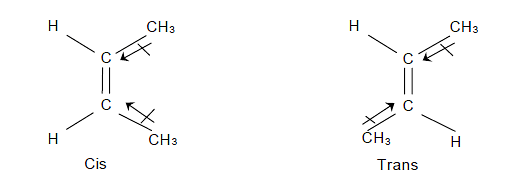Bond Parameters | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| What are Bond Parameters? |

|
| Bond Length |

|
| Bond Enthalpy |

|
| Bond angle |

|
| Bond order |

|
| Formal Charge |

|
| Dipole Moment |

|
| Resonance in Chemical Bonding |

|
What are Bond Parameters?
Covalent bonds can be characterized on the basis of several bond parameters such as bond length, bond angle, bond order, and bond energy (also known as bond enthalpy). These bond parameters offer insight into the stability of a chemical compound and the strength of the chemical bonds holding its atoms together.
Bond Length
 Bond Length
Bond Length
- Bond length or bond distance is defined as the equilibrium internuclear separation distance of the bonded atoms in a molecule.
- It is measured by spectroscopic, X-ray diffraction and electron diffraction techniques.
- In the case of covalent bond, the contribution from each atom is called covalent radius of that atom.
- In the periodic table the covalent radius increases down a group and they decrease for s and p-block elements from left to right across a period.
- The bond length decreases from single covalent bond to triple covalent bond.
Factors affecting the bond length
- Size of the atoms: The bond length increases with increase in the size of the atoms. For example, bond lengths of H–X are in the order:
HI > HBr > HCl > HF - Multiplicity of bond: The bond length decreases with the multiplicity of the bond. Thus, bond length of carbon-carbon bonds are in the order:
C ≡ C < C = C < C – C - Type of hybridisation: As an sorbital is smaller in size, greater the s-character, shorter is the hybrid orbital and hence shorter is the bond length. For example,
Bond lengths : sp3 C–H > sp2 C–H > sp C–H
s-character : (25%) (33%) (50%) - Resonance and delocalisation: C-C bond length is 1.54Å and C = C bond length is 1.34 Å but in benzene, due to resonance, the carbon-carbon bond is neither single nor double but intermediate between single and double and same holds for bond length and is equal to 1.39 Å.
Bond Enthalpy
- Bond enthalpy, i.e., the strength of a chemical bond, is measured as the bond dissociation enthalpy.
- It is the enthalpy required to break a particular bond in one mole of gaseous molecule.
Factors affecting Bond energy
(i) Size of the atoms: Greater the size of the atoms, greater is the bond length and less is the bond dissociation enthalpy, i.e., less is the bond strength.
(ii) Multiplicity of bonds: For the bond between the same two atoms, greater is the multiplicity of the bond, greater is the bond dissociation enthalpy. This is firstly because atoms come closer and secondly, the number of bonds to be broken is more. For example, bond dissociation enthalpies of H2, O2 and N2 are in the order: H–H < O = O < N ≡ N
(iii) Number of lone pairs of electrons present: Greater the number of lone pairs of electrons present on the bonded atoms greater is the repulsion between the atoms and hence less is the bond dissociation enthalpy. For example, for a few single bonds, we have
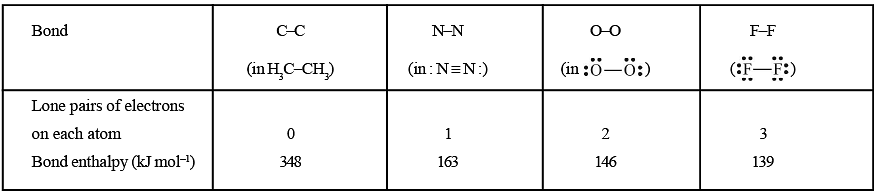 Bond Enthalpies
Bond Enthalpies
Bond Length
The equilibrium distance between the centres of the nuclei of the two bonded atoms is called its bond length.
Factors affecting the bond length
- Size of the atoms: The bond length increases with increase in the size of the atoms. For example, bond lengths of H–X are in the order:
HI > HBr > HCl > HF - Multiplicity of bond: The bond length decreases with the multiplicity of the bond. Thus, bond length of carbon-carbon bonds are in the order:
C ≡ C < C = C < C – C - Type of hybridisation: As an sorbital is smaller in size, greater the s-character, shorter is the hybrid orbital and hence shorter is the bond length. For example,
Bond lengths : sp3 C–H > sp2 C–H > sp C–H
s-character : (25%) (33%) (50%) - Resonance and delocalisation: C-C bond length is 1.54Å and C = C bond length is 1.34 Å but in benzene, due to resonance, the carbon-carbon bond is neither single nor double but intermediate between single and double and same holds for bond length and is equal to 1.39 Å.
Bond angle
The angle between the lines representing the directions of the bonds, i.e., the orbitals containing the bonding electrons is called the bond angle.
Factors Affecting Bond Angle
- Hybridization: The bond angle depends on the state of hybridization of the central atom.
Hybridization sp3 sp2 sp
Bond angle 109028' 1200 1800
Example CH4 BCl3 BeCl2
It is observed that as s-character increases in the hybrid bond, the bond angle increases. - Lone pair repulsion: The bond angle is affected by the presence of a lone pair of electrons at the central atom. A lone pair of electrons at the central atom always tries to repel the shared pair (bonded pair) of electrons. Due to this, the bonds are displaced slightly inside resulting in a decrease of bond angle. The bond angle in NH3 is 107° and the bond angle in H2O is 105° even though N-atom and O-atom undergo sp3 hybridization.
- Electronegativity: If the electronegativity of the central atom decreases, the bond angle decreases. In case the central atom remains the same, bond angle increases with a decrease ill the electronegativity of the surrounding atoms.
 Example of Bond Angles
Example of Bond Angles
Bond order
- In the Lewis representation of a molecule or ion, the number of bonds presents between two atoms is called bond order. For example, the bond orders of a few molecules are given below:

- For odd electron molecules, as the three electron bond is considered as equivalent to half covalent bond, bond order can be fractional also. For example, Lewis's structure of NO is
 Lewis's structure of NO
Lewis's structure of NO - It is observed that with increase in bond order, bond enthalpy increases while bond length decreases.
Formal Charge
The formal charge on an atom in a molecule or ion is defined as the difference between the number of valence electrons of that atom in the free state and the number of electrons assigned to that atom in the Lewis structure, assuming that in each shared pair of electrons, the atom has one electron of its own and the lone pair on it belongs to it completely.
Thus, it can be calculated as follows: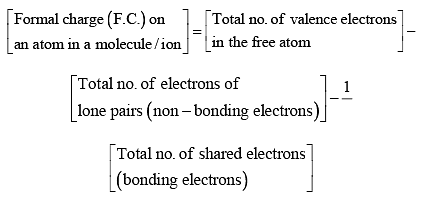 Formal Charge FormulaExample:
Formal Charge FormulaExample:
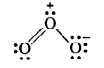
Calculate the formal charge on each O-atom of the O3 molecule.
Sol. Lewis structure of O3 is: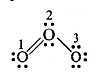 The atoms have been numbered as 1, 2 and 3.
The atoms have been numbered as 1, 2 and 3.
Formal charge on end O-atom numbered 1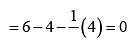
The formal charge on the central O-atom numbered 2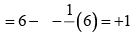 Formal charge on end O-atom numbered 3
Formal charge on end O-atom numbered 3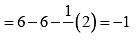 Hence, we represent O3 along with formal charges as:
Hence, we represent O3 along with formal charges as:
Example:
Write the formal charges on atoms in (i) carbonate ion (ii) nitrite ion.
Sol. (i) Lewis structure of CO32– ion is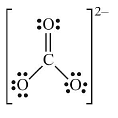 Formal charge on C atom
Formal charge on C atom
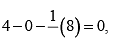 Formal charge on double bonded O atom
Formal charge on double bonded O atom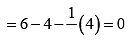 Formal charge on single bonded O atom
Formal charge on single bonded O atom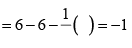 (ii) Lewis structure of NO2– ion is
(ii) Lewis structure of NO2– ion is 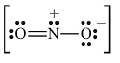 Formal charge on N atom
Formal charge on N atom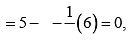 Formal charge on double bonded O atom
Formal charge on double bonded O atom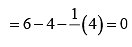 Formal charge on single bonded O atom
Formal charge on single bonded O atom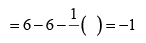 Significance of formal charge:
Significance of formal charge:
The main advantage of the calculation of formal charges is that it helps to select the most stable structure, i.e., the one with least energy out of the different possible Lewis structures. The most stable is the one which has the smallest formal charges on the atoms.
Dipole Moment
Dipole Moment
- If a covalent bond is formed between two dissimilar atoms, eg. A and B, one of the atoms (A or B) must be more electronegative than the other. If A is more electronegative than the shared pair of electrons is drawn near A leaving a positive charge on B and hence making the molecule dipolar (A–B+). The percentage of polar character is given in terms of dipole moment (μ).
- The dipole moment is defined as the product of electric charge q and the distance r between the two atoms of a polar molecule. (μ = e × d)
- Dipole moment is a vector quantity with direction same as that of the line joining positive and negative centres.
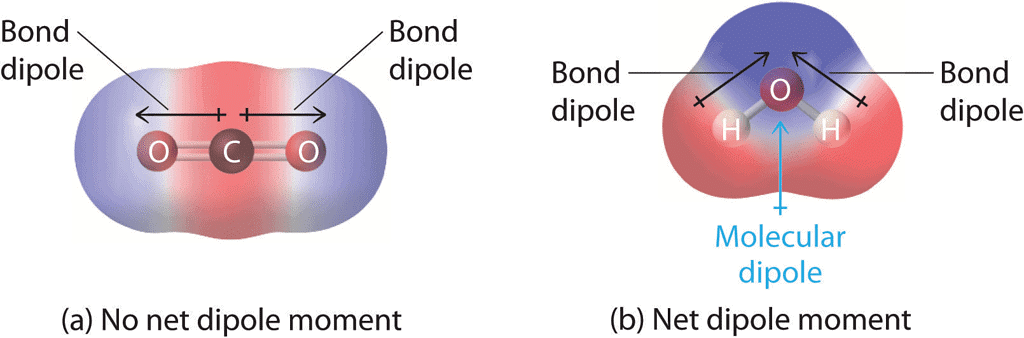
- Thus molecules having dipole moment (μ = 0) are called non-polar molecules and molecules with > 0 are polar. The greater is μ, the greater is the polarity.
- For polyatomic molecules with two or more bonds, the net dipole moment is the resultant of vector addition of individual moments.
- While expressing dipole moments, generally charge is given in electrostatic units (esu) and distance in angstrom units (1Aº =10–10 m). Thus dipole moment of an electron separated from unit positive charge by a distance 1Aº would be (4.80 × 10–10 esu) × (10-8 cm) = 4.8 × 10–18 esu cm = 4.8 Debye.
Applications of Dipole Moment
- The dipole moment helps predict the geometry of the molecule.
- The dipole moment helps in determining the polarity.
- Dipole moment can distinguish between symmetrical and non-symmetrical molecules. eg. CO2 has a 0 dipole moment as it is symmetrical whereas H2O has a dipole moment of 1.85D.

- Cis and trans isomers can be distinguished by dipole moments, usually, cis isomers have higher dipole moments and hence higher polarity e.g.

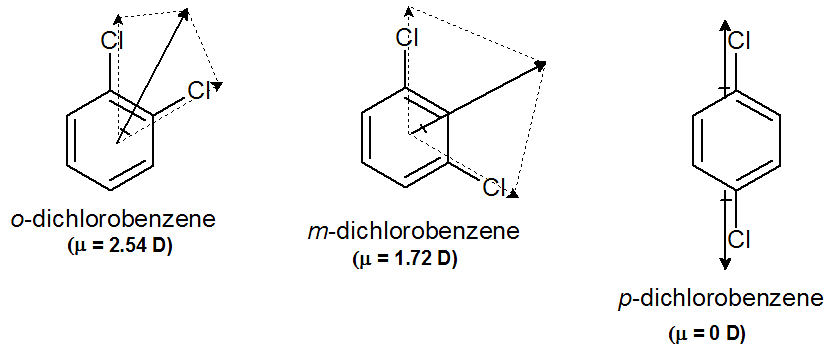
- The dipole moment is greatest for ortho isomer, zero for para isomer, and less than that of ortho for meta isomer. o > m > p. e.g.

- The ionic character can be determined by using dipole moment
E.g. experimental dipole moment for HCl is 1.03 and μ = r X eas r = 1.26 and, e = 4.8 × 10-10 esu,μ = 6.05 Debye. Thus we can say % ionic character
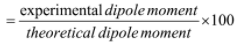
- Hybridization can be determined by dipole moment for eg.
- If a molecule AB2 has μ=0, the s orbitals used by A (z < 21) must be sp hybridised e.g. BeF2
- If a molecule AB3 has μ=0, the s orbitals used by A (z < 21) must be sp2 hybridised e.g. BF3
- If a molecule AB4 has μ =0, the s orbitals used by A (z < 21) must be sp3 hybridised e.g. CCl4
Resonance in Chemical Bonding
There are molecules and ions for which drawing a single Lewis structure is not possible. For example, we can write two structures of O3.
In (A) the oxygen-oxygen bond on the left is a double bond and the oxygen-oxygen bond on the right is a single bond. In B the situation is just the opposite. The experiment shows, however, that the two bonds are identical.
Therefore neither structure A nor B can be correct. One of the bonding pairs in ozone is spread over the region of all three atoms rather than localized on a particular oxygen-oxygen bond. This delocalized bonding is a type of chemical bonding in which bonding pair of electrons are spread over a number of atoms rather than localized between two.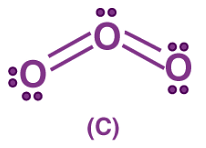
Structures (A) and (B) are called resonating or canonical structures and (C) is the resonance hybrid. This phenomenon is called resonance, a situation in which more than one canonical structure can be written for a species. The chemical activity of an atom is determined by the number of electrons in its valence shell. With the help of the concept of chemical bonding, one can define the structure of a compound and is used in many industries for manufacturing products in which the true structure cannot be written at all.
Some other examples:
- CO32– ion
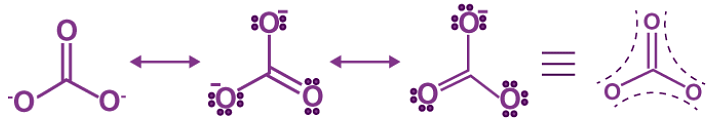
- Carbon-oxygen bond lengths in carboxylate ion are equal due to resonance.
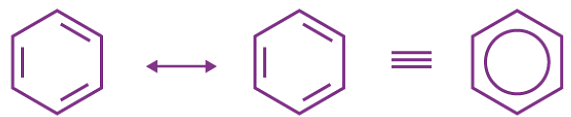
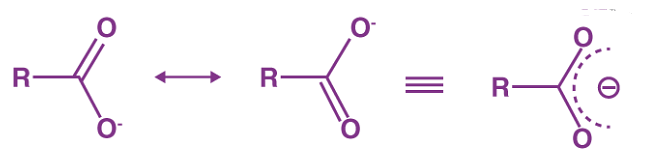 - Benzene
- Benzene
 -Vinyl Chloride
-Vinyl Chloride

The difference in the energies of the canonical forms and resonance hybrid is called resonance stabilization energy.
|
127 videos|244 docs|87 tests
|
FAQs on Bond Parameters - Chemistry Class 11 - NEET
| 1. What is bond length? |  |
| 2. How is bond enthalpy defined? |  |
| 3. What is bond angle? |  |
| 4. How is bond order calculated? |  |
| 5. What is formal charge in chemical bonding? |  |

|
Explore Courses for NEET exam
|

|