Class 10 Exam > Class 10 Notes > Chapter Notes for Class 10 > Chapter Notes: Periodic Classification of Elements
Periodic Classification of Elements Class 10 Notes Science
| Table of contents |

|
| Introduction |

|
| Dobereiner’s Traids |

|
| Newland’s Law of Octaves |

|
| Mendeleev’s Periodic Table |

|
| Modern Periodic Table |

|
Introduction
- Matter exists in various forms, including elements, compounds, and mixtures.
- Elements are the most basic units of matter, consisting of only one type of atom. Examples include sodium (Na), magnesium (Mg), and gold (Au). There are currently 118 known elements, each with its own distinct properties.
- To make studying these elements easier, scientists group them based on similarities in their properties.
Dobereiner’s Traids
- When elements were arranged in the order of increasing atomic masses, groups of three elements (known as traids), having similar chemical properties are obtained.
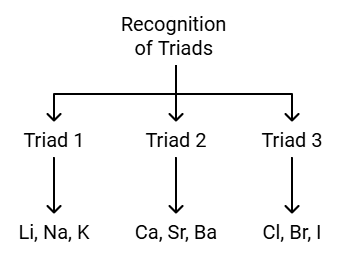
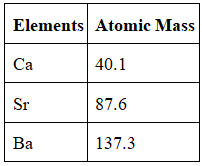
- The atomic mass of the middle element of the triad was roughly the average of the atomic masses of the other two elements.
Limitations of Dobereiner’s Traids
- Limited Scope: Dobereiner's Triads only applied to a small number of elements (around 20) and couldn't explain the properties of many other elements.
- Inconsistent Pattern: The triad pattern of elements with similar chemical properties did not always hold, especially for heavier elements, leading to exceptions and inconsistencies.
- No Atomic Structure Explanation: Dobereiner's triads did not consider atomic structure or the role of atomic number in determining element properties, which later led to the development of the periodic table based on atomic number.
Question for Chapter Notes: Periodic Classification of ElementsTry yourself: Which of the following is a limitation of Dobereiner's traids?View Solution
Newland’s Law of Octaves
- Newland arranged the known elements by increasing atomic mass and observed that the properties of every 8th element were similar to those of the 1st element.
- This pattern was likened to musical octaves, hence it was named the 'Law of Octaves.'
- For example, the properties of lithium (Li) and sodium (Na) were found to be similar.
 Limitations of Newland’s Law of Octaves
Limitations of Newland’s Law of Octaves
- It was applicable upto calcium (for lighter elements only).
- Properties of new discovered elements did not fit into the law of octave.
- To fit elements into his table, Newlands put even two elements together in one slot and that too in the column of unlike elements having very different properties.
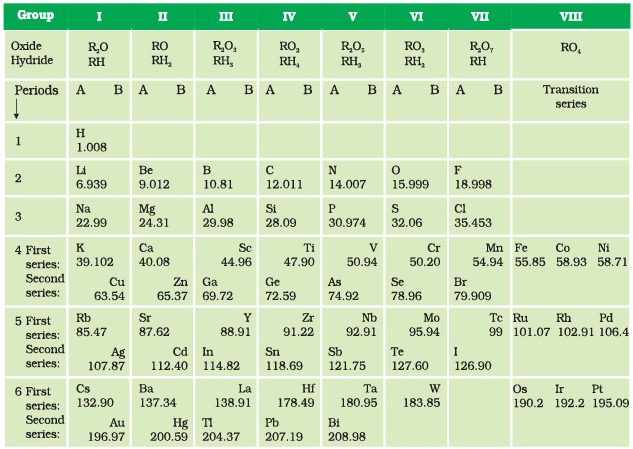
Mendeleev’s Periodic Table
- When elements are arranged in the order of increasing atomic masses, the element with similar properties occur at regular intervals.
- The properties of elements are a periodic function of their atomic masses.
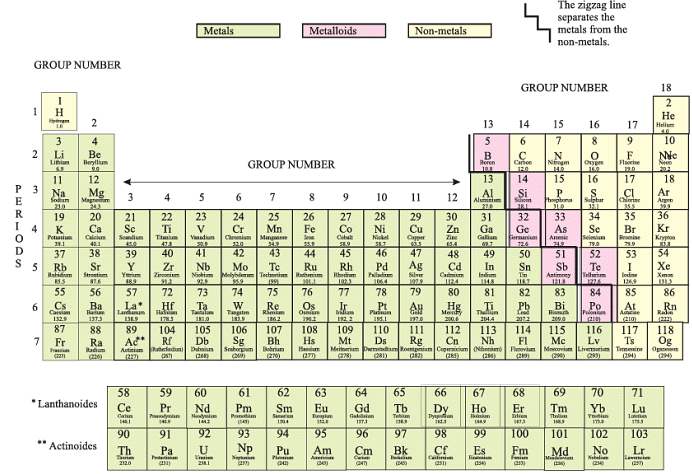
- Mendleev’s periodic table is based on the chemical properties of elements. It contains 7 periods (horizontal rows) and 8 groups (vertical columns).
Merits of Mendeleev’s Periodic Table
- Some gaps were left for the undiscovered elements like gallium (Ga), Scandium (Sc) and Germanium (Ge).
- Predict properties of elements on the basis of their positions in the periodic table.
- Accommodate noble gases when they were discovered without disturbing the original arrangement.
Modern Periodic Table
- Atomic number of an element is a more fundamental property than its atomic mass.
- According to the Modern Periodic law: The properties of elements are a periodic function of their atomic number.
- All the anomalies of Mendeleev’s classification disappear.
Explanation of the Anomalies by Modern Periodic Table
- Explanation for the position of isotopes (Same atomic number put at one place in the same group).
- Cobalt with atomic number 27 came first and nickel (28) should come later.
- Unlike atomic masses, atomic number is always a whole number, so there is no element between hydrogen and helium.
- Atomic Number: It is denoted by Z and equal to the number of protons in the nucleus of an atom.
- Modern Periodic table has 18 vertical columns known as ‘groups’ and 7 horizontal rows known as ‘periods’.
- Elements with same number of valence electrons are placed in the same group.
Example:
Li : 2, 1
Na : 2, 8, 1
K : 2, 8, 8, 1 - Outermost or valence shell in all the three contains 1 electron. These elements have been placed in the same group.
- Number of shells increases as we go down the group.
- Elements with same number of occupied shells are placed in same period.
- For example, Li (2, 1); Be (2, 2); B (2, 3), C (2, 4), N(2, 5). These elements have same number of shells (two).
- Each period marks a new electronic shell getting filled.
- Number of elements placed in a particular period depends upon the fact that how electrons are filled into various shell.
- Maximum number of electrons that can be filled in a shell is given by 2n2 where n is shell number.
Example:
K shell n = 1 or 2n2 = 2(1)2 = 2 (First period has 2 elements.)
L shell n = 2 or 2n2 = 2(2)2 = 8 (Second period has 8 elements.) - Position of an element in the periodic table tells us its chemical reactivity.
- Valence electron determine the kind and number of bonds formed by the element.
Question for Chapter Notes: Periodic Classification of ElementsTry yourself: Which law of classification was based on the analogy between elements and musical octaves?View Solution
Trends in the Modern Periodic Table
- Valency: No. of valence electrons present in the outermost shell of its atom. On moving from left to right in each period, the valency of elements increases from 1 to 4 and then decreases to 0.
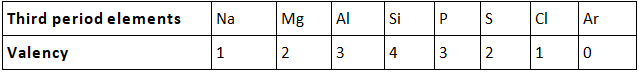 Valency remains the same down in a group.
Valency remains the same down in a group.
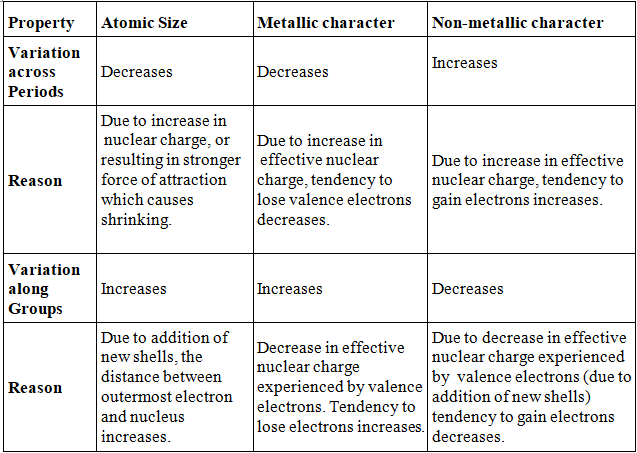
- Atomic size: Atomic size refers to the radius of an atom. It may be visualized as the distance between the centre of the nucleus and the outermost shell.
- Atomic size or radius of an atom decreases as we move from left to right in a period because due to large +ve charge on the nucleus, the electrons are pulled in more close to the nucleus and size decreases.
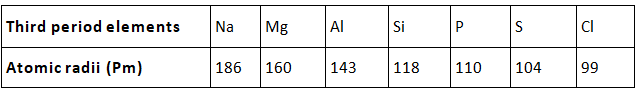
- Atomic size increases as we move down the group because new shells are being added and this increases the distance between nucleus and outermost electron.
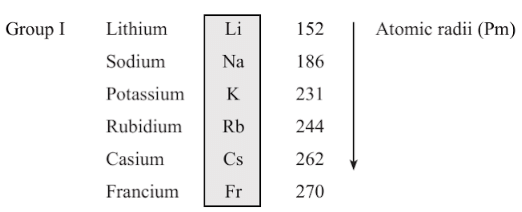
Metallic Character
- Metallic character means the tendency of an atom to lose electron.
- Metals occupy the left hand side of the periodic table.
- On moving left to right in a period, the metallic character of an element decreases because the effective nuclear charge increases. It means tendency to lose electron decreases.
- Metals are electropositive as they tend to lose electrons while forming bonds.
- Metallic character increases as we go down a group as the effective nuclear charge is decreasing.
Non-metallic Character
- Non-metals are electronegative as they tend to form bonds by gaining electrons.
- Non-metals occupies the right side of the periodic table.
- Non-metallic character increases across a period because due to increase in effective nuclear charge that means tendency to gain electron increase.
- Non-metallic character decreases as we move down a group due to decrease in effective nuclear charge experienced by the valence electron thus the tendency to gain electron decreases.In
- the middle of periodic table we have semi-metals or metalloid because they exhibit some properties of metals and non-metals.
- Oxides of metals are basic in nature while oxides of non-metals are acidic in nature.
Atomic size, Metallic character and non-metallic according to Periodic table
The document Periodic Classification of Elements Class 10 Notes Science is a part of the Class 10 Course Chapter Notes for Class 10.
All you need of Class 10 at this link: Class 10
FAQs on Periodic Classification of Elements Class 10 Notes Science
| 1. What is the periodic table and why is it important in chemistry? |  |
Ans.The periodic table is a systematic arrangement of chemical elements organized by their atomic number, electron configuration, and recurring chemical properties. It is important because it provides a useful framework for understanding the relationships between different elements, predicting their chemical behavior, and facilitating the study of chemistry.
| 2. Who developed the first periodic table and what was its significance? |  |
Ans.Dmitri Mendeleev is credited with developing the first widely recognized periodic table in 1869. Its significance lies in its ability to organize elements based on their properties and atomic masses, leading to the prediction of undiscovered elements and the establishment of periodic law.
| 3. How are elements categorized in the periodic table? |  |
Ans.Elements in the periodic table are categorized into groups (columns) and periods (rows). Groups contain elements with similar chemical properties, while periods correspond to the number of electron shells. This classification helps in understanding the behavior of elements and their compounds.
| 4. What are the major groups of elements in the periodic table? |  |
Ans.The major groups of elements in the periodic table include metals, nonmetals, and metalloids. Metals are typically good conductors of heat and electricity, nonmetals are poor conductors and have varied properties, and metalloids possess characteristics of both metals and nonmetals.
| 5. How does the periodic table help in predicting chemical reactions? |  |
Ans.The periodic table helps in predicting chemical reactions by allowing scientists to identify trends in element properties, such as electronegativity, ionization energy, and atomic radius. By understanding these trends, chemists can anticipate how different elements will interact and form compounds.
Related Searches
















