Chemistry of Transition Metals & Bonding in Molecules | Chemistry Optional Notes for UPSC PDF Download
Chemistry of Transition Metals
What is a transition metal?
The terms transition metal (or element) and d block element are sometimes used as if they mean the same thing. They don't - there's a subtle difference between the two terms. We'll explore d block elements first:
You will remember that when you are building the Periodic Table and working out where to put the electrons using the Aufbau Principle, something odd happens after argon. At argon, the 3s and 3p levels are full, but rather than fill up the 3d levels next, the 4s level fills instead to give potassium and then calcium. Only after that do the 3d levels fill. The elements in the Periodic Table which correspond to the d levels filling are called d block elements.
The first row of these is shown in the shortened form of the Periodic Table below:
The electronic structures of the d block elements shown are:
You will notice that the pattern of filling is not entirely tidy! It is broken at both chromium and copper.
Not all d block elements count as transition metals!
A transition metal is one that forms one or more stable ions which have incompletely filled d orbitals. On the basis of this definition, scandium and zinc do not count as transition metals - even though they are members of the d block.
- Scandium has the electronic structure [Ar] 3d14s2. When it forms ions, it always loses the 3 outer electrons and ends up with an argon structure. The Sc3+ ion has no d electrons and so does not meet the definition.
- Zinc has the electronic structure [Ar] 3d104s2. When it forms ions, it always loses the two 4s electrons to give a 2+ ion with the electronic structure [Ar] 3d10. The zinc ion has full d levels and does not meet the definition either.
By contrast, copper, [Ar] 3d104s1, forms two ions. In the Cu+ ion the electronic structure is [Ar] 3d10. However, the more common Cu2+ ion has the structure [Ar] 3d9. Copper is definitely a transition metal because the Cu2+ ion has an incomplete d level.
Transition metal ions
- Here you are faced with one of the most irritating facts in chemistry at this level! When you work out the electronic structures of the first transition series (from scandium to zinc) using the Aufbau Principle, you do it on the basis that the 3d orbitals have higher energies than the 4s orbitals.
- That means that you work on the assumption that the 3d electrons are added after the 4s ones. However, in all the chemistry of the transition elements, the 4s orbital behaves as the outermost, highest energy orbital. When these metals form ions, the 4s electrons are always lost first.
The 4s electrons are lost first in forming d-block ions
To write the electronic structure for Co2+:
The 2+ ion is formed by the loss of the two 4s electrons.
To write the electronic structure for V3+: The 4s electrons are lost first followed by one of the 3d electrons.
The 4s electrons are lost first followed by one of the 3d electrons.
Variable oxidation state (number)
One of the key features of transition metal chemistry is the wide range of oxidation states (oxidation numbers) that the metals can show. It would be wrong, though, to give the impression that only transition metals can have variable oxidation states. For example, elements like Sulfur or nitrogen or chlorine have a very wide range of oxidation states in their compounds - and these obviously aren't transition metals. However, this variability is less common in metals apart from the transition elements. Of the familiar metals from the main groups of the Periodic Table, only lead and tin show variable oxidation state to any extent.
Examples of variable oxidation states in the transition metals
- Iron: Iron has two common oxidation states (+2 and +3) in, for example, Fe2+ and Fe3+. It also has a less common +6 oxidation state in the ferrate(VI) ion, FeO42-.
- Manganese: Manganese has a very wide range of oxidation states in its compounds. For example:

Explaining the variable oxidation states in the transition metals
We'll look at the formation of simple ions like Fe2+ and Fe3+. When a metal forms an ionic compound, the formula of the compound produced depends on the energetics of the process. On the whole, the compound formed is the one in which most energy is released. The more energy released, the more stable the compound.
There are several energy terms to think about, but the key ones are:
- The amount of energy needed to ionize the metal (the sum of the various ionization energies)
- The amount of energy released when the compound forms. This will either be lattice enthalpy if you are thinking about solids, or the hydration enthalpies of the ions if you are thinking about solutions.
The more highly charged the ion, the more electrons you have to remove and the more ionization energy you will have to provide. But off-setting this, the more highly charged the ion, the more energy is released either as lattice enthalpy or the hydration enthalpy of the metal ion.
Thinking about a typical non-transition metal (calcium)
- The formula for Calcium chloride is CaCl2. Why is that? If you tried to make CaCl, (containing a Ca+ ion), the overall process is slightly exothermic. By making a Ca2+ ion instead, you have to supply more ionization energy, but you get out lots more lattice energy. There is much more attraction between chloride ions and Ca2+ ions than there is if you only have a 1+ ion. The overall process is very exothermic. Because the formation of CaCl2 releases much more energy than making CaCl, then CaCl2 is more stable - and so forms instead.
- What about CaCl3? This time you have to remove yet another electron from calcium. The first two come from the 4s level. The third one comes from the 3p. That is much closer to the nucleus and therefore much more difficult to remove. There is a large jump in ionization energy between the second and third electron removed. Although there will be a gain in lattice enthalpy, it is not anything like enough to compensate for the extra ionization energy, and the overall process is very endothermic. It definitely is not energetically sensible to make CaCl3!
Thinking about a typical transition metal (iron)
Here are the changes in the electronic structure of iron to make the 2+ or the 3+ ion. The 4s orbital and the 3d orbitals have very similar energies. There is not a huge jump in the amount of energy you need to remove the third electron compared with the first and second. The figures for the first three ionization energies (in kJ mol-1) for iron compared with those of calcium are:
The 4s orbital and the 3d orbitals have very similar energies. There is not a huge jump in the amount of energy you need to remove the third electron compared with the first and second. The figures for the first three ionization energies (in kJ mol-1) for iron compared with those of calcium are:

There is an increase in ionization energy as you take more electrons off an atom because you have the same number of protons attracting fewer electrons. However, there is much less increase when you take the third electron from iron than from calcium.
In the iron case, the extra ionization energy is compensated more or less by the extra lattice enthalpy or hydration enthalpy evolved when the 3+ compound is made. The net effect of all this is that the overall enthalpy change is not vastly different whether you make, say, FeCl2 or FeCl3. That means that it is not too difficult to convert between the two compounds.
The formation of complex ions
What is a complex ion?
A complex ion has a metal ion at its center with a number of other molecules or ions surrounding it. These can be considered to be attached to the central ion by coordinate (dative covalent) bonds (in some cases, the bonding is actually more complicated). The molecules or ions surrounding the central metal ion are called ligands. Simple ligands include water, ammonia and chloride ions.
What all these have got in common is active lone pairs of electrons in the outer energy level. These are used to form co-ordinate bonds with the metal ion.
Some examples of complex ions formed by transition metals
Other metals also form complex ions - it is not something that only transition metals do. Transition metals do, however, form a very wide range of complex ions.
The formation of colored compounds
The diagrams show approximate colors for some common transition metal complex ions.
The origin of color in the transition metal ions
When white light passes through a solution of one of these ions, or is reflected off it, some colors in the light are absorbed. The color you see is how your eye perceives what is left. Attaching ligands to a metal ion has an effect on the energies of the d orbitals. Light is absorbed as electrons move between one d orbital and another. This is explained in detail on another page.
Catalytic Activity
Transition metals and their compounds are often good catalysts. A few of the more obvious cases are mentioned below, but you will find catalysis explored in detail elsewhere on the site (follow the link after the examples). Transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to adsorb other substances on to their surface and activate them in the process. All this is explored in the main catalysis section.
Iron in the Haber Process
The Haber Process combines hydrogen and nitrogen to make ammonia using an iron catalyst.
Nickel in the hydrogenation of C=C bonds
This reaction is at the heart of the manufacture of margarine from vegetable oils. However, the simplest example is the reaction between ethene and hydrogen in the presence of a nickel catalyst.
Transition metal compounds as catalysts
Vanadium(V) oxide in the Contact Process
At the heart of the Contact Process is a reaction which converts Sulfur dioxide into Sulfur trioxide. Sulfur dioxide gas is passed together with air (as a source of oxygen) over a solid vanadium(V) oxide catalyst.
Iron ions in the reaction between persulfate ions and iodide ions
Persulphate ions (peroxodisulphate ions), S2O82-, are very powerful oxidizing agents. Iodide ions are very easily oxidized to iodine. And yet the reaction between them in solution in water is very slow. The reaction is catalyzed by the presence of either iron(II) or iron(III) ions.
Bonding in Molecules
Introduction
Filling atomic orbitals requires a set number of electrons. The s-block is composed of elements of Groups I and II, the alkali and alkaline earth metals (sodium and calcium belong to this block). Groups XIII through XVIII comprise of the p-block, which contains the nonmetals, halogens, and noble gases (carbon, nitrogen, oxygen, fluorine, and chlorine are common members). Transition metals reside in the d-block, between Groups III and XII. If the following table appears strange, or if the orientations are unclear, please review the section on atomic orbitals.

The key thing to remember about electronic configuration is that the most stable noble gas configuration is ideal for any atom. Forming bonds are a way to approach that configuration. In particular, the transition metals form more lenient bonds with anions, cations, and neutral complexes in comparison to other elements. This is because the d orbital is rather diffused (the f orbital of the lanthanide and actinide series more so).
Neutral-Atom Electron Configurations
Counting through the periodic table is an easy way to determine which electrons exist in which orbitals. As mentioned before, by counting protons (atomic number), you can tell the number of electrons in a neutral atom. Organizing by block quickens this process. For example, if we were interested in determining the electronic organization of Vanadium (atomic number 23), we would start from hydrogen and make our way down the the Periodic Table).
1s (H, He), 2s (Li, Be), 2p (B, C, N, O, F, Ne), 3s (Na, Mg), 3p (Al, Si, P, S, Cl, Ar), 4s (K, Ca), 3d (Sc, Ti, V).
If you do not feel confident about this counting system and how electron orbitals are filled, please see the section on electron configuration.
Referring to the periodic table below confirms this organization. We have three elements in the 3d orbital. Therefore, we write in the order the orbitals were filled.
1s2 2s2 2p6 3s2 3p6 4s2 3d3
or
[Ar] 4s2 3d3.
The neutral atom configurations of the fourth period transition metals are in Table 8.2.2.
Chromium and copper appear anomalous. Take a brief look at where the element Chromium (atomic number 24) lies on the Periodic Table (Figure 8.2.1). The electronic configuration for chromium is not [Ar] 4s23d4 but instead it is [Ar] 4s13d5. This is because the half-filled 3d manifold (with one 4s electron) is more stable than a partially filled d-manifold (and a filled 4s manifold). You will notice from Table 8.2.2 that the copper exhibits a similar phenomenon, although with a fully filled d-manifold.
Oxidation States of Transition Metal Ions
When considering ions, we add or subtract negative charges from an atom. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient method. An atom that accepts an electron to achieve a more stable configuration is assigned an oxidation number of -1. The donation of an electron is then +1. When a transition metal loses electrons, it tends to lose it's s orbital electrons before any of its d orbital electrons. For more discussion of these compounds form, see formation of coordination complexes.
Solved Examples
Example 1: Write the electronic configurations of:
- neutral iron,
- iron(II) ion, and
- iron(III) ion.
Ans: The atomic number of iron is 26 so there are 26 protons in the species.
- Fe: [Ar] 4s2 3d6
- Fe2+: [Ar] 3d6
- Fe3+: [Ar] 3d5
Note that the s-orbital electrons are lost first, then the d-orbital electrons.
Example 2: Determine the more stable configuration between the following pair:
- [Kr] 5s2 4d6 vs. [Kr] 5s1 4d7
- Ag1+ vs. Ag2+
Ans:
- This describes Ruthenium. There is only one 5s electron.
- Once-oxidized silver ([Kr] 4d10) is more stable than twice- ([Kr] 4d9).
Multiple Oxidation States
Most transition metals have multiple oxidation states, since it is relatively easy to lose electron(s) for transition metals compared to the alkali metals and alkaline earth metals. Alkali metals have one electron in their valence s-orbital and their ions almost always have oxidation states of +1 (from losing a single electron). Similarly, alkaline earth metals have two electrons in their valences s-orbitals, resulting in ions with a +2 oxidation state (from losing both). However, transitions metals are more complex and exhibit a range of observable oxidation states due primarily to the removal of d-orbital electrons. The following chart describes the most common oxidation states of the period 3 elements.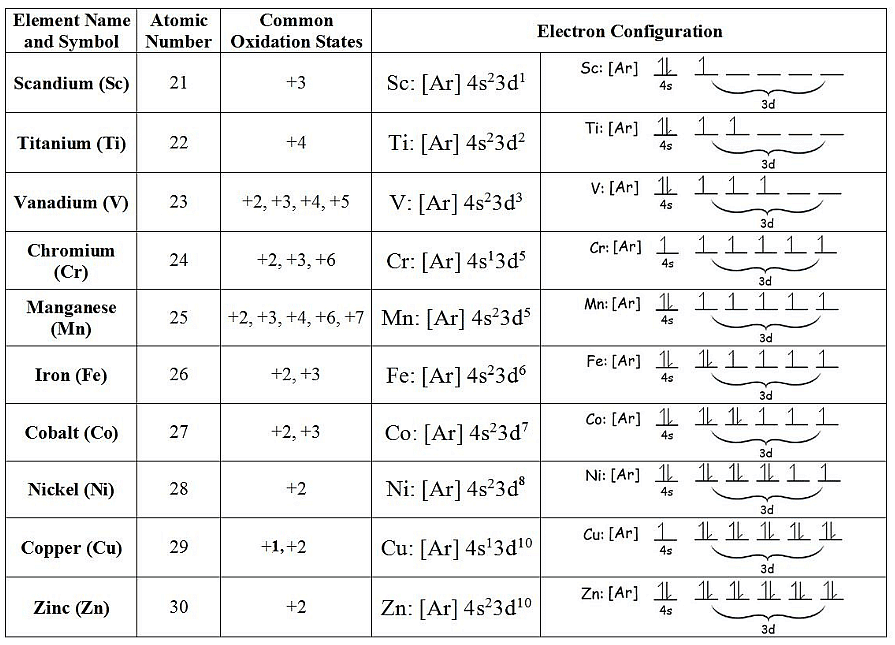
Scandium is one of the two elements in the first transition metal period which has only one oxidation state (zinc is the other, with an oxidation state of +2). All the other elements have at least two different oxidation states. Manganese, which is in the middle of the period, has the highest number of oxidation states, and indeed the highest oxidation state in the whole period since it has five unpaired electrons (see table below).
To help remember the stability of higher oxidation states for transition metals it is important to know the trend: the stability of the higher oxidation states progressively increases down a group. For example, in group 6, (chromium) Cr is most stable at a +3 oxidation state, meaning that you will not find many stable forms of Cr in the +4 and +5 oxidation states. By contrast, there are many stable forms of molybdenum (Mo) and tungsten (W) at +4 and +5 oxidation states.
Solved Examples
Example 1: What makes zinc stable as Zn2+? What makes scandium stable as Sc3+?
Ans: Zinc has the neutral configuration [Ar]4s23d10. Losing 2 electrons does not alter the complete d orbital. Neutral scandium is written as [Ar]4s23d1. Losing 3 electrons brings the configuration to the noble state with valence 3p6.
Example 2: Why is iron almost always Fe2+ or Fe3+?
Ans: Iron is written as [Ar]4s23d6. Losing 2 electrons from the s-orbital (3d6) or 2 s- and 1 d-orbital (3d5) electron are fairly stable oxidation states.
Example 3: Write manganese oxides in a few different oxidation states. Which ones are possible and/or reasonable?
Ans: Although Mn+2 is the most stable ion for manganese, the d-orbital can be made to remove 0 to 7 electrons. Compounds of manganese therefore range from Mn(0) as Mn(s), Mn(II) as MnO, Mn(II,III) as Mn3O4, Mn(IV) as MnO2, or manganese dioxide, Mn(VII) in the permanganate ion MnO4-, and so on.
Oxidation State of Transition Metals in Compounds
When given an ionic compound such as AgCl , you can easily determine the oxidation state of the transition metal. In this case, you would be asked to determine the oxidation state of silver (Ag). Since we know that chlorine (Cl) is in the halogen group of the periodic table, we then know that it has a charge of -1, or simply Cl-. In addition, by seeing that there is no overall charge for AgCl , (which is determined by looking at the top right of the compound, i.e., AgCl#, where # represents the overall charge of the compound) we can conclude that silver ( Ag ) has an oxidation state of +1. This gives us Ag+ and Cl-, in which the positive and negative charge cancels each other out, resulting with an overall neutral charge; therefore +1 is verified as the oxidation state of silver (Ag).
Solved Examples
Example 1: Determine the oxidation state of cobalt in CoBr2.
Ans: Similar to chlorine, bromine (Br) is also a halogen with an oxidation charge of -1 ( Br−). Since there are two bromines each with a charge of -1. In addition, we know that CoBr2 has an overall neutral charge, therefore we can conclude that the cation (cobalt), Co must have an oxidation state of +2 to neutralize the -2 charge from the two bromine anions.
Example 2: What is the oxidation state of zinc in ZnCO3. (Note: the CO3 anion has a charge state of -2)
Ans: Knowing that CO3 has a charge of -2 and knowing that the overall charge of this compound is neutral, we can conclude that zinc has an oxidation state of +2. This gives us Zn2+ and CO−23, in which the positive and negative charges from zinc and carbonate will cancel with each other, resulting in an overall neutral charge expected of a compound.
Polyatomic Transition Metal Ions
Consider the manganese (Mn) atom in the permanganate (MnO−4) ion. Since oxygen has an oxidation state of -2 and we know there are four oxygen atoms. In addition, this compound has an overall charge of -1; therefore the overall charge is not neutral in this example. Thus, since the oxygen atoms in the ion contribute a total oxidation state of -8, and since the overall charge of the ion is -1, the sole manganese atom must have an oxidation state of +7. This gives us Mn7+ and 4O2−, which will result as MnO−4.
This example also shows that manganese atoms can have an oxidation state of +7, which is the highest possible oxidation state for the fourth period transition metals.
Manganese: A Case Study
Manganese is widely studied because it is an important reducing agent in chemical analysis and is also studied in biochemistry for catalysis and in metallurgy in fortifying alloys. In plants, manganese is required in trace amounts; stronger doses begin to react with enzymes and inhibit some cellular function. Due to manganese's flexibility in accepting many oxidation states, it becomes a good example to describe general trends and concepts behind electron configurations.Figure 8.2.2: (left) A rough fragment of lustrous silvery metal Some of the Lascaux cave paintings use manganese-based pigments.
Electron configurations of unpaired electrons are said to be paramagnetic and respond to the proximity of magnets. Fully paired electrons are diamagnetic and do not feel this influence. Manganese, in particular, has paramagnetic and diamagnetic orientations depending on what its oxidation state is.
Mn2O3 is manganese(III) oxide with manganese in the +3 state. 4 unpaired electrons means this complex is paramagnetic.
[Ar]4s03d4
MnO2 is manganese(IV) oxide, where manganese is in the +4 state. 3 unpaired electrons means this complex is less paramagnetic than Mn3+.
[Ar]4s03d3
KMnO4 is potassium permanganate, where manganese is in the +7 state with no electrons in the 4s and 3d orbitals.
[Ar]4s03d0
Since the 3p orbitals are all paired, this complex is diamagnetic.
Summary
Oxidation states of transition metals follow the general rules for most other ions, except for the fact that the d orbital is degenerated with the s orbital of the higher quantum number. Transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose electrons to other atoms and ions. These resulting cations participate in the formation of coordination complexes or synthesis of other compounds.
FAQs on Chemistry of Transition Metals & Bonding in Molecules - Chemistry Optional Notes for UPSC
| 1. What are transition metals? |  |
| 2. What is the significance of variable oxidation states in transition metal ions? |  |
| 3. How do transition metal compounds act as catalysts? |  |
| 4. How do transition metals form complex ions? |  |
| 5. How does the bonding in transition metal compounds differ from other molecules? |  |

|
Explore Courses for UPSC exam
|

|

 Figure 8.2.2: (left) A rough fragment of lustrous silvery metal Some of the Lascaux cave paintings use manganese-based pigments.
Figure 8.2.2: (left) A rough fragment of lustrous silvery metal Some of the Lascaux cave paintings use manganese-based pigments.















