Classification & Nomenclature of Haloalkanes & Haloarenes | Chemistry Class 12 - NEET PDF Download
What are Haloalkanes?
Haloalkanes, also known as alkyl halides, are organic compounds where one or more hydrogen atoms in an alkane molecule are replaced by halogen atoms such as fluorine, chlorine, bromine, or iodine. These compounds are characterized by the presence of carbon-halogen bonds.
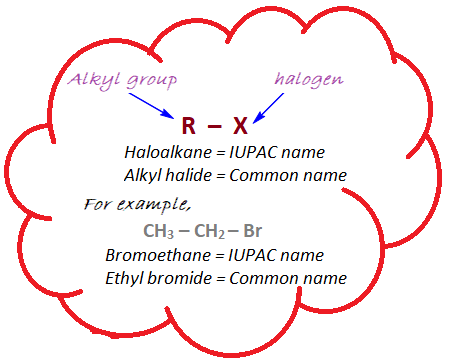 Haloalkanes, also known as alkyl halides, are organic compounds in which one or more hydrogen atoms in an alkane molecule are replaced by halogen atoms such as fluorine, chlorine, bromine, or iodine. The general formula for haloalkanes is R-X, where R represents an alkyl group (a chain of carbon atoms) and X represents a halogen atom.
Haloalkanes, also known as alkyl halides, are organic compounds in which one or more hydrogen atoms in an alkane molecule are replaced by halogen atoms such as fluorine, chlorine, bromine, or iodine. The general formula for haloalkanes is R-X, where R represents an alkyl group (a chain of carbon atoms) and X represents a halogen atom.
- For example, chloroform (CHCl3) and bromobutane (C4H9Br) are common examples of haloalkanes.
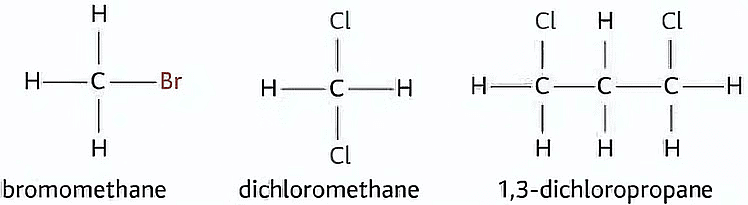 Examples of Haloalkanes
Examples of Haloalkanes
- Haloalkanes are widely used in organic synthesis, as solvents, and in some cases as pharmaceuticals or pesticides.
What are Haloarenes?
Haloarenes, are organic compounds derived from aromatic hydrocarbons like benzene, with one or more hydrogen atoms substituted by halogen atoms. They are structurally similar to haloalkanes but have their halogen atoms directly attached to an aromatic ring.
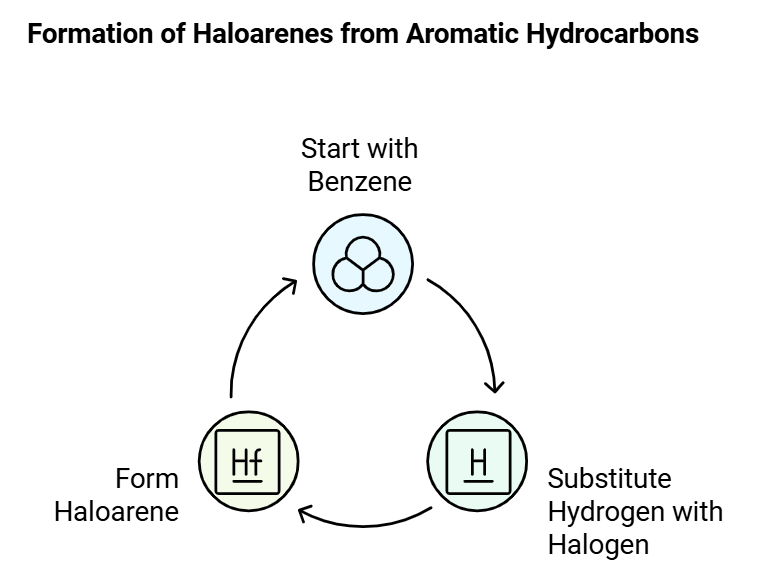
The main distinction between haloalkanes and haloarenes lies in their origin; haloalkanes originate from open-chain hydrocarbons (alkanes), while haloarenes are derived from aromatic hydrocarbons.
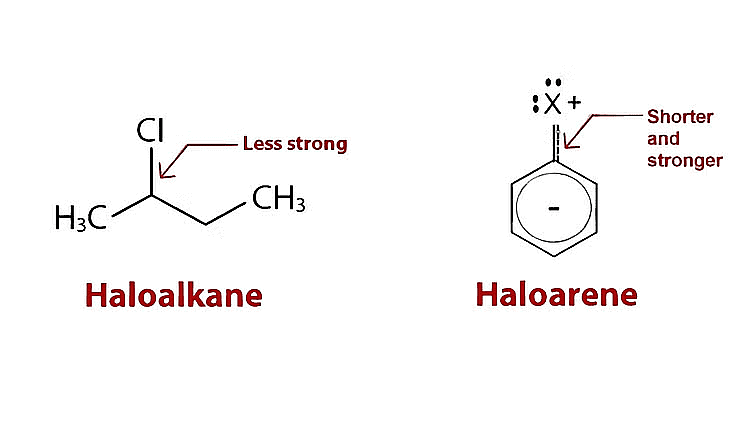 Haloalkanes and Haloarenes
Haloalkanes and Haloarenes
- The general formula for haloarenes is Ar-X, where Ar represents an aryl group (a benzene ring or its derivatives) and X represents a halogen atom.
- Common examples of haloarenes include chlorobenzene (C6H5Cl) and bromobenzene (C6H5Br).
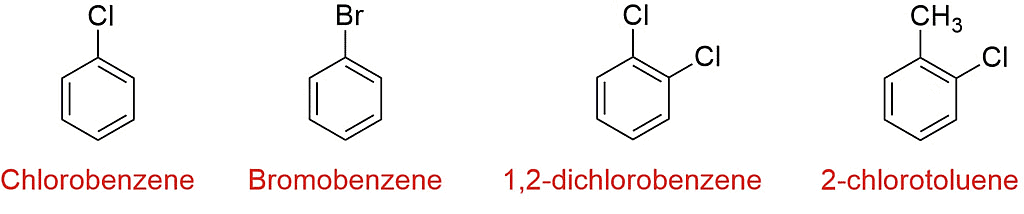 Examples of Haloarenes
Examples of Haloarenes
- Haloarenes are also used in organic synthesis and can be found in some pharmaceuticals, agrochemicals, and dyes.
Classification of Haloalkanes and Haloarene
Haloalkanes and Haloarenes can be classified based on:1. Number of halogen atoms in the molecule.
2. sp3 hybridized carbon-halogen bond.
3. sp2 hybridized carbon-halogen bond.
1. Classification based on the number of Halogen Atoms
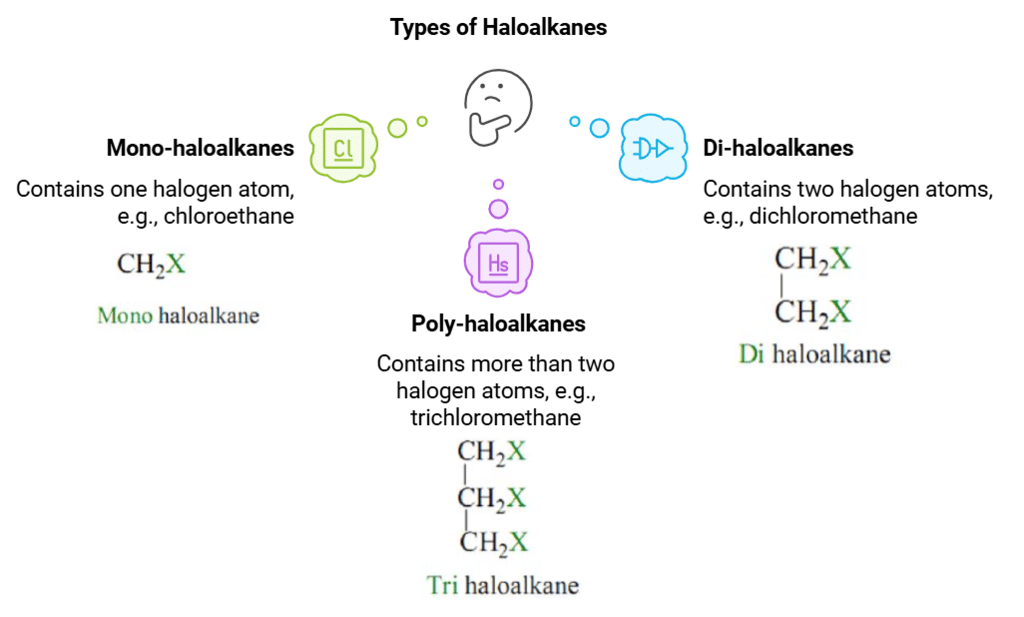
Based on the number of halogen atoms, they can be divided into mono, di, or poly (tri, tetra, and so on) compounds of haloalkanes and haloarenes.
(a) Haloalkanes
- Mono-haloalkanes: These are haloalkanes that contain only one halogen atom. Examples include chloroethane (C2H5Cl) and bromopropane (C3H7Br).
- Di-haloalkanes: These are haloalkanes that contain two halogen atoms. The halogen atoms can be the same or different. Examples include dichloromethane (CH2Cl2) and dibromobutane (C4H9Br2).
- Poly-haloalkanes: These are haloalkanes that contain more than two halogen atoms. Examples include trichloromethane (chloroform, CHCl3) and tetrachloroethane (C2H2Cl4).
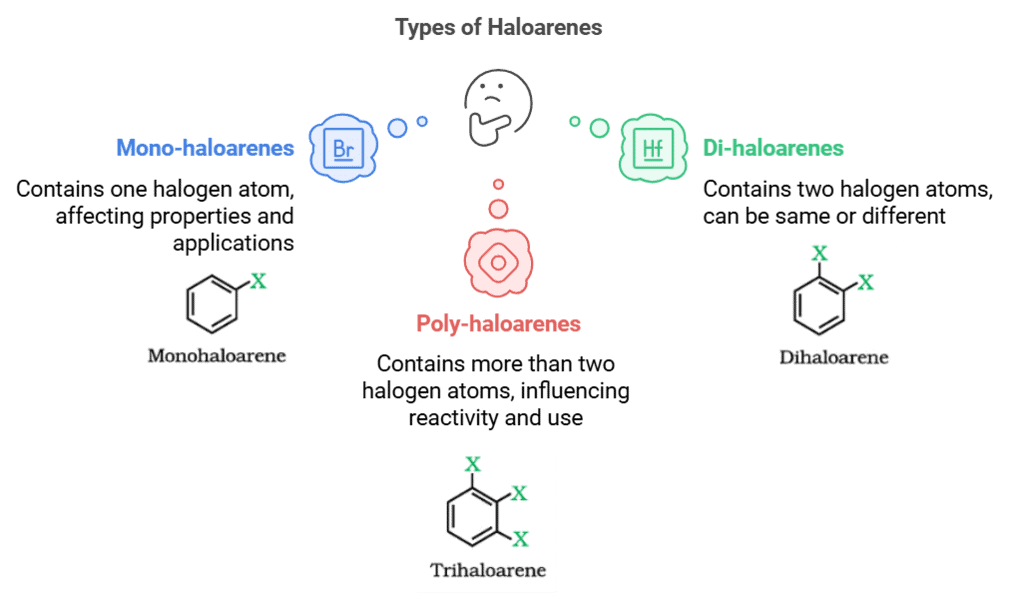
(b) Haloarenes
The number and position of halogen atoms in haloarenes affect their physical properties, reactivity, and applications.
- Mono-haloarenes: These are haloarenes that contain only one halogen atom. Examples include chlorobenzene (C6H5Cl) and fluorobenzene (C6H5F).
- Di-haloarenes: These are haloarenes that contain two halogen atoms. The halogen atoms can be the same or different. Examples include dichlorobenzene (C6H4Cl2) and dibromobenzene (C6H4Br2).
- Poly-haloarenes: These are haloarenes that contain more than two halogen atoms. Examples include trichlorobenzene (C6H3Cl3) and tetrabromobenzene (C6H2Br4).
2. Classification based on halogen attached to sp3 hybridized carbon
Alkyl halides are haloalkanes and haloarenes where the halogen is attached to an sp3 hybridized carbon atom. They are commonly referred to as alkyl halides. These compounds can have fluorine, chlorine, bromine, or iodine as the halogen, and the halogen atom is directly bonded to a carbon atom that has sp3 hybridization.
Alkyl halides can be further classified based on the nature of the carbon atom to which the halogen is attached. The classifications are as follows:
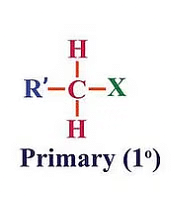
(a) Primary (1°) haloalkanes/haloarenes
These are alkyl halides where the carbon atom bonded to the halogen atom is directly attached to only one other carbon atom. Primary (1°) haloalkanes/haloarenes examples include chloroethane (C2H5Cl) and chlorobenzene (C6H5Cl).
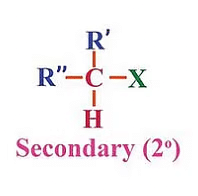
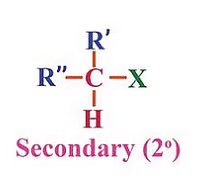
 (b) Secondary (2°) haloalkanes/haloarenes:
(b) Secondary (2°) haloalkanes/haloarenes:
These are alkyl halides where the carbon atom bonded to the halogen atom is directly attached to two other carbon atoms. Secondary (2°) haloalkanes/haloarenes examples include 2-chloropropane (C3H7Cl) and 1-chloro-4-methylbenzene (C7H7Cl).
(c) Tertiary (3°) haloalkanes/haloarenes
These are alkyl halides where the carbon atom bonded to the halogen atom is directly attached to three other carbon atoms. Tertiary (3°) haloalkanes/haloarenes examples include 2-chloro-2-methylpropane (C4H9Cl) and 1-chloro-2,4,6-trimethylbenzene (C9H11Cl).
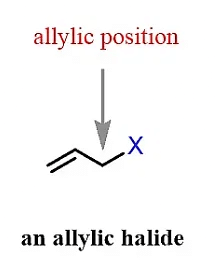
(d) Allylic Halide
The halogen atom is attached to an sp3 hybridized carbon which is adjacent to C=C (double bond or sp2 hybridized carbon)
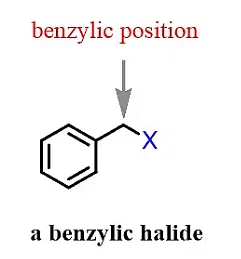
(e) Benzylic Halide
The halogen is attached to a sp3 hybridized carbon which is attached to a benzene ring.
3. Classification based on halogen attached to sp2 hybridized carbon
Compounds in which the halogen (such as chlorine, bromine, or iodine) is attached to a sp2 hybridized carbon atom are known as vinyl or aryl halides. The carbon atom in these compounds is part of a double bond or a triple bond, resulting in the sp2 hybridization.
(a) Vinyl Halides
These compounds are formed when a halogen atom is attached to an sp2 hybridized carbon atom present next to a carbon-carbon double bond (C=C).
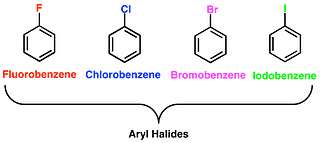
(b) Aryl Halides
This class of compounds is formed when the halogen group is bonded to an sp2 hybridized atom of carbon in an aromatic ring.
Nomenclature of Haloalkanes
The nomenclature of alkyl halides follows the same basic IUPAC rules that are discussed for naming alkanes. The only difference here is that one (or more) of the substituents is a halogen and its naming is a little modified.
Let’s first recall the nomenclature rules by naming the following alkane:
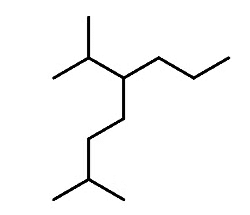
Step 1: Determine the parent chain. In this case, the longest chain consists of eight carbons, making the parent chain octane. If there are two chains of equal length, priority is given to the one with more substituents.
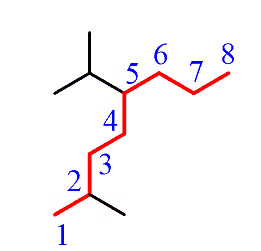
Step 2: Identify the substituents. Here, we have a methyl group and an isopropyl group.
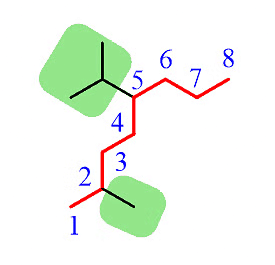
Step 3: Number the parent chain to assign the lowest possible numbers to the substituents.
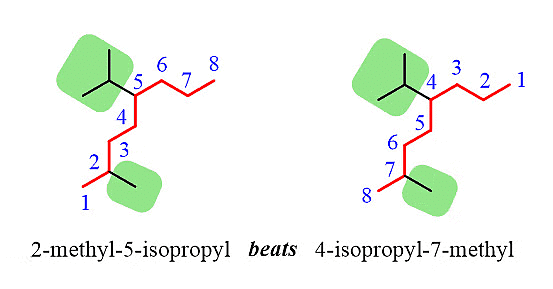
Step 4: Combine the parent chain and substituents, arranging the substituents in alphabetical order.
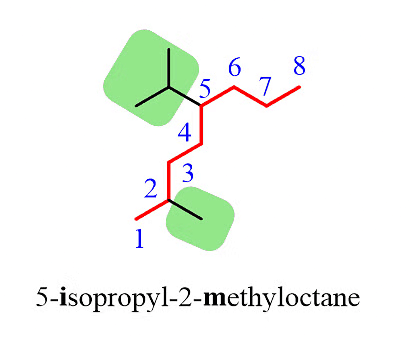
Note: It's important to note that despite the isopropyl group being at position 5, it is placed before the methyl group at position 2 because the substituents are ordered alphabetically.
Additionally, it's worth mentioning that only the "iso" prefix is considered for alphabetical priority. Other common groups are based on the first letter of the carbon chain and not the prefix.
Alkyl Halides
(a) In alkyl halides, the halogen atom is considered as an alkyl substituent without any priority over carbon atoms. Therefore, when numbering the parent chain, the goal is still to assign the lowest possible number(s) to the substituents.
(b) The main distinction in naming alkyl halides lies in the modification of the suffix "ine" to "o." For instance, instead of using "ine" as the suffix, we use "o" to indicate the presence of the halogen. This can be seen in examples such as 2-bromo, 4-chloro, 3-iodo, and 5-fluoro.
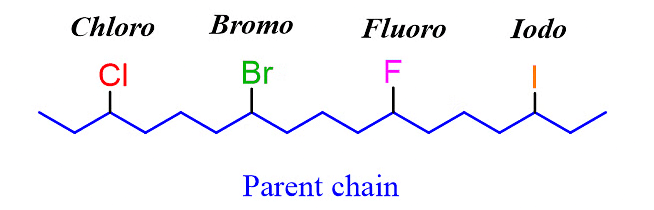
Let’s name the following alkyl halide:
Step 1. Determine the parent chain by identifying the longest chain of carbon atoms. In this case, the longest chain consists of eight carbons, making it the parent chain known as octane.
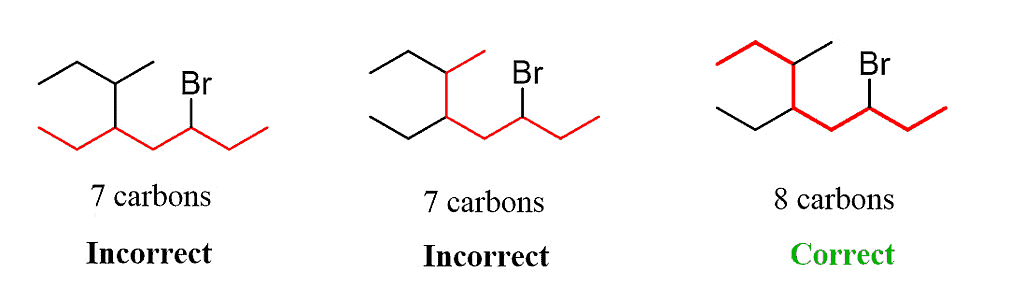
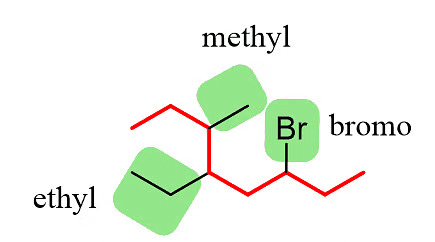
Step 2: Identify the substituents. In this example, there are three substituents, which include two alkyl groups and a halide.
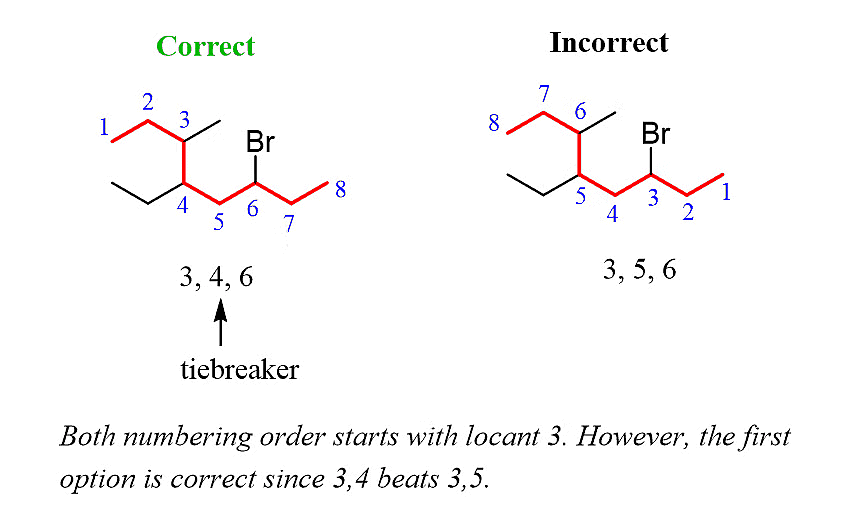
Step 3: Number the parent chain, assigning the lowest possible numbers to the substituents. If there is a tie for the first locant, compare the second and then the third locants.
Step 4: Combine the parent chain and substituents, arranging the substituents in alphabetical order.
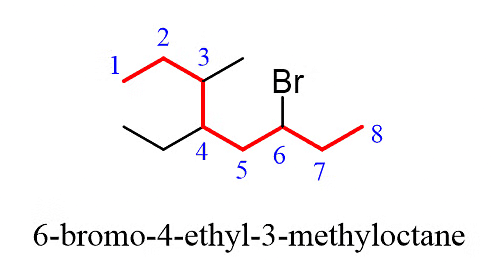
Once again, it is important to note that in the final compound name, the substituents are placed in alphabetical order. Even though the halogen (Br) is at position 6, it is still listed before the other substituents.
Note: The IUPAC name of any halogen derivative is always written as one word.
IUPAC Name of Halogen Derivatives
Solved Examples on Nomenclature of Haloalkanes
Q1: What is the IUPAC name for the compound CH3CH(Cl)CH(Br)CH3?
Ans: The IUPAC name for CH3CH(Cl)CH(Br)CH3 is 2-Bromo-3-chlorobutane.
Solution: The parent chain is a four-carbon chain, which is called butane.
- The bromine atom (Br) is attached to the second carbon atom, so it is named 2-bromo.
- The chlorine atom (Cl) is attached to the third carbon atom, so it is named 3-chloro.
Therefore, combining these names, we get the IUPAC name 2-Bromo-3-chlorobutane for the given compound.
Q2: What is the IUPAC name for the compound CHF2CBrClF?
Ans: The IUPAC name for CHF2CBrClF is 1-Bromo-1-chloro-1,2,2-trifluoroethane.
Solution: The parent chain is ethane, as it consists of two carbon atoms connected by a single bond.
- The bromine atom (Br) is attached to the first carbon atom, so it is named 1-bromo.
- The chlorine atom (Cl) is also attached to the first carbon atom, so it is named 1-chloro.
- There are two fluorine atoms (F) attached to the second carbon atom, so it is named 1,2-difluoro.
- The prefix "tri" is added to indicate the presence of three fluorine atoms.
Therefore, combining these names, we get the IUPAC name 1-Bromo-1-chloro-1,2,2-trifluoroethane for the given compound.
Nomenclature of Haloarenes
- Naming Monohaloarenes: The common name and IUPAC name of Aryl halides are the same. These are named by prefixing “halo” to the parent name of the aromatic hydrocarbon. For example:
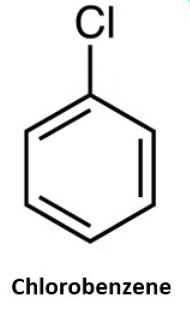
- Naming Substituted Haloarenes: If there is more than one halogen substituent on the aromatic ring, then, according to IUPAC nomenclature, the relative positions of the substituents are indicated by mathematical numerals.
In the common system, the relative position of two groups is shown by prefixes o–,m–and p– that indicate ortho, meta, or para positions.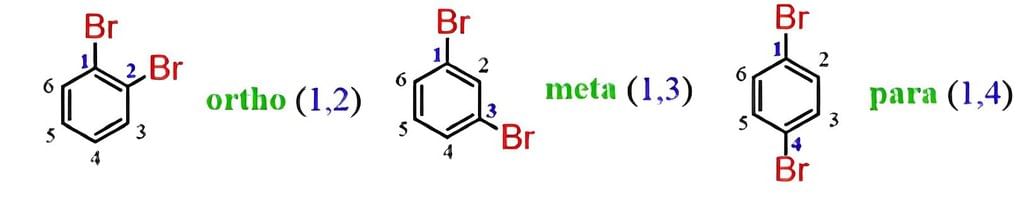 The halogen atom acts as a substituent rather than as a functional group. Hence, they do not give characteristic suffixes to the hydrocarbon. They rank the lowest in the priority list of functional groups.
The halogen atom acts as a substituent rather than as a functional group. Hence, they do not give characteristic suffixes to the hydrocarbon. They rank the lowest in the priority list of functional groups.
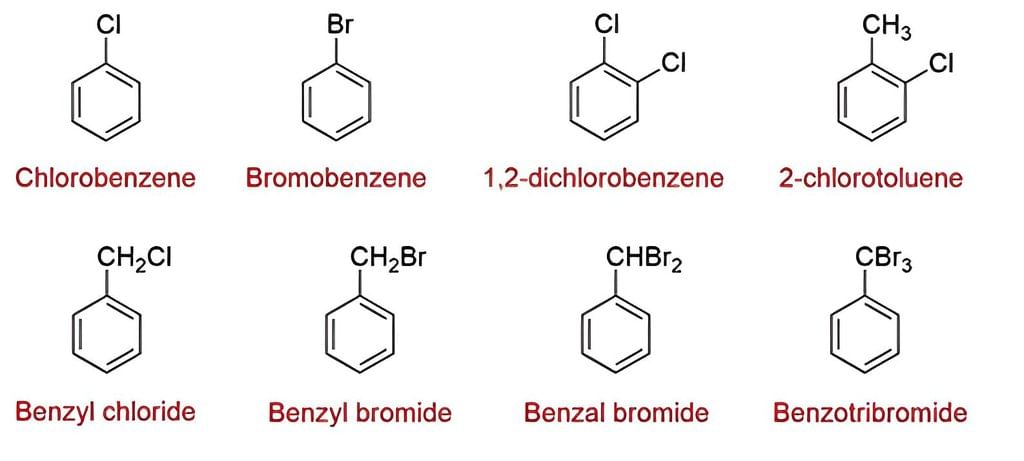 Nomenclature of Haloarenes
Nomenclature of Haloarenes
Solved Examples on Nomenclature of Haloarenes
Q.1. What is the IUPAC name of the compound CH3C(p-ClC6H4)2CH(Br)CH3?
The IUPAC name for CH3C(p-ClC6H4)2CH(Br)CH3 is 2-Bromo-3,3-bis(4-chlorophenyl)butane.
Solution:
- The parent chain is butane, consisting of four carbon atoms connected by single bonds.
- The bromine atom (Br) is attached to the second carbon atom, so it is named 2-bromo.
- There are two substituents, which are (p-ClC6H4) groups. The p-ClC6H4 group is a 4-chlorophenyl group, indicating a chlorine atom (Cl) attached to the fourth carbon of a benzene ring. Since there are two of these groups, it is named bis(4-chlorophenyl).
- The methyl group (CH3) is attached to the third carbon atom, indicating it is named as 3-methyl.
- Therefore, combining these names, we get the IUPAC name 2-Bromo-3,3-bis(4-chlorophenyl)butane for the given compound.
Q.2. Which of the following is not the correct IUPAC name for the compound shown?
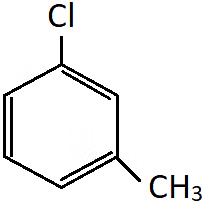
a) 1-Chloro-3-methylbenzene
b) 3-Chloromethylbenzene
c) 3-Chlorotoluene
d) m-Chlorotoluene
Answer: m-Chlorotoluene
Solution: All the names given are appropriate for the shown compound, but m-chlorotoluene is the common name and not the IUPAC name.
Factors Affecting Bond Polarity & Reactivity of Alkyl Halides
The C-X bond in haloalkanes and haloarenes exhibits a highly polar nature due to the electronegativity difference between the carbon atom and the halogen atom. The halogen, being more electronegative, attracts electron density towards itself, resulting in a partial negative charge on the halogen atom and a partial positive charge on the carbon atom. This bond polarity significantly impacts the reactivity of these compounds.
Several factors affect the polarity of the C-X bond and consequently influence the reactivity:
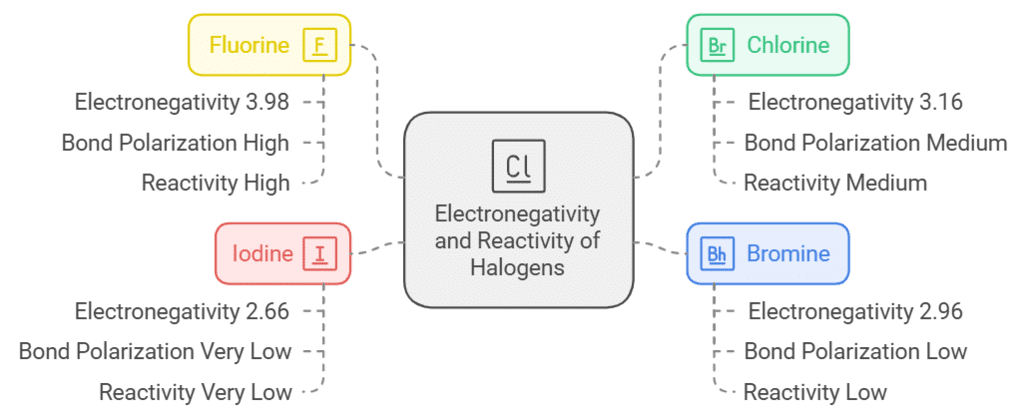
Electronegativity:
- The electronegativity of the halogen atoms determines the degree of polarization in the bond.
- Fluorine, the most electronegative halogen( F(3.98) > Cl(3.16) > Br(2.96) > I(2.66)), induces the highest degree of polarization in the C-X bond, making it highly reactive.
- Chlorine, bromine, and iodine follow in decreasing order of electronegativity, resulting in decreasing bond polarity and reactivity.
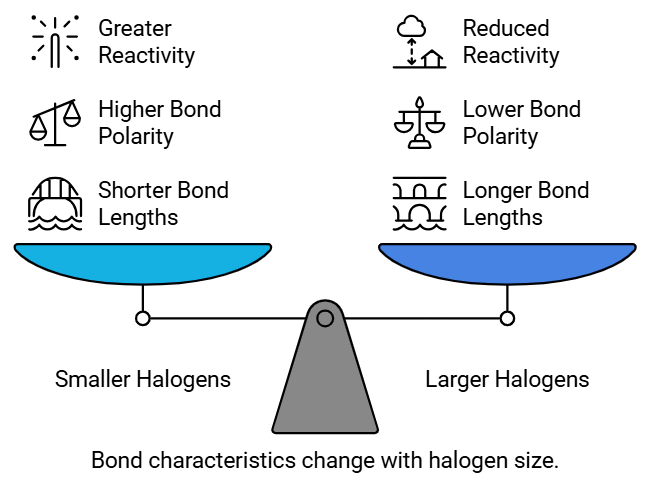
Bond Length :
- The length of the C-X bond plays a role in bond polarity.
- As we move down the halogen group, the size of the halogen atom increases.
- (F < Cl < Br < I). This increase in atomic size leads to longer bond lengths in haloalkanes and haloarenes. Longer bond lengths result in decreased bond polarity and reactivity.
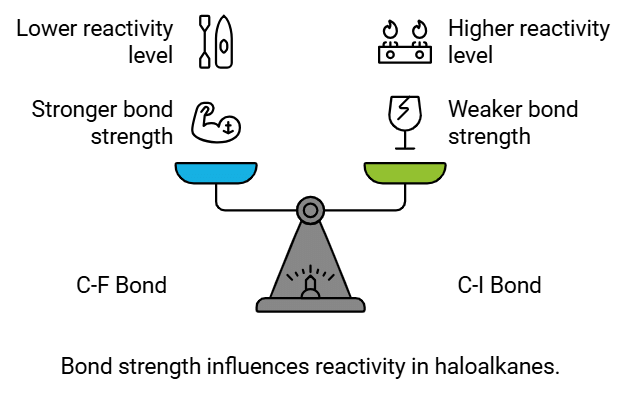
Bond Strength:
- The strength of the C-X bond, indicated by bond enthalpy, impacts the reactivity of haloalkanes and haloarenes.
- Stronger bonds require higher energy input to break, making them less reactive. The C-F bond, being the shortest and strongest among the C-X bonds, exhibits the lowest reactivity.
- On the other hand, the C-I bond, being the longest and weakest, shows the highest reactivity.
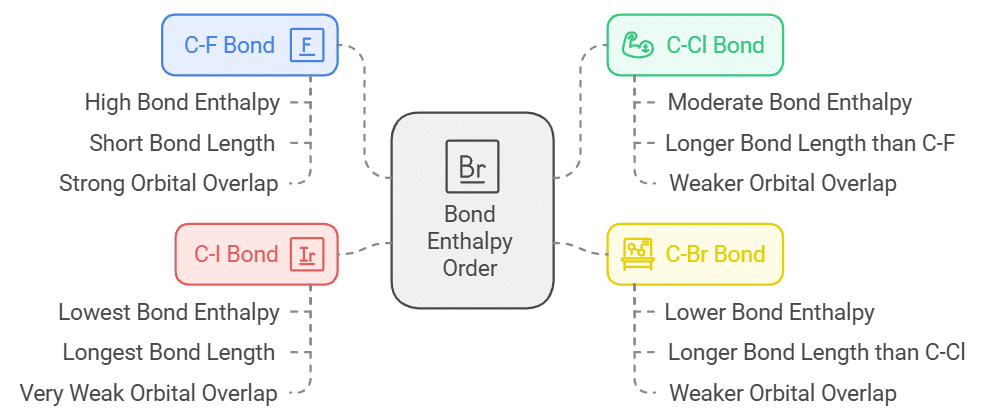
Bond Enthalpy Order:
- The nature of the C-X bond depends upon bond enthalpy order. The size of the carbon and fluorine atom is very similar so the orbitals overlap (2p-2p overlap) into one another.
- This leads to the formation of a very strong bond. In C-I the atomic size of iodine is very large in comparison to carbon atom so the orbital interaction is very weak.
- This results in the formation of weak bond strength. We can conclude that less the bond length stronger will be the bond. Hence, the bond length of C-F is 1.39 Ao.
- The stronger the bond, the amount of energy required increases to break that bond. Therefore, C-F has the highest bond enthalpy. The bond enthalpy order is: C − F > C − Cl > C − Br > C – I
Dipole Moment:
- The dipole moment of the C-X bond reflects its polarity. A higher dipole moment indicates a greater separation of charges and increased bond polarity.
- It occurs due to the separation of positive and negative charges. It is the product of both charge and the distance between them. Bond dipole (μ) is given by the formula μ = q × d.
- The order of dipole moment in C-X is CH3Cl > CH3F > CH3Br > CH3I.
- In haloalkanes and haloarenes, the dipole moment follows the trend CH3Cl > CH3F > CH3Br > CH3I.
Dipole moments of haloalkanes are: CH3F − 1.847D, CH3Cl − 1.860 D, CH3Br − 1.830 D, CH3I − 1.636 D
- Can you notice the abnormal order of the dipole moment in the case where CH3Cl > CH3F?
Even though fluorine is more electronegative than Chlorine but the C-F bond (139 pm) is shorter than the C-Cl bond C − Cl (178 pm). Thus, the dipole moment will be lower in the case of CH3F in comparison to CH3Cl. - Understanding these factors helps predict and explain the reactivity patterns of haloalkanes and haloarenes. Compounds with stronger C-X bonds and higher bond polarity tend to exhibit lower reactivity, while those with weaker bonds and lower polarity display higher reactivity.
Some Important Questions
Q.1. Monohalo, dihalo, trihalo, and tetrahalo are types of haloalkanes and haloarenes based on the
a) type of halogen atom
b) number of halogen atoms
c) nature of carbon atom
d) hybridisation of the C atom to which halogen is bonded
Answer: b)
Explanation: Haloalkanes may be classified as mono, di, tri, tetra, and so on depending on the number of halogen atoms present in their structure.
Q.2. Which of the following compounds contains an allylic carbon?
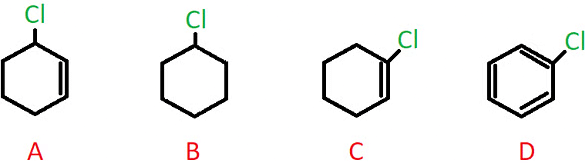
a) A
b) B
c) C
d) D
Answer: a)
Explanation: A sp3 hybridized C atom present adjacent to a C-C double bond is called an allylic carbon, and when the halogen atom is bonded to this carbon, it is called an allylic halide.
Q.3. Which of the following categories does the compound shown belong to?
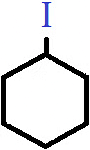
a) Primary haloalkane
b) Secondary haloalkane
c) Tertiary haloalkane
d) Haloarene
Answer: b)
Explanation: The compound shown is cyclohexyl iodide, in which the halogen atom is bonded to an alicyclic alkyl group. Since there are effectively two alkyl groups attached to the carbon bonded to the halogen atom, it is classified as secondary or 2° cyclo alkyl halide.
Q.4. What is the nature of the circled C atom in the following compound?
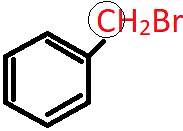
a) sp2 hybridised
b) allylic
c) benzylic
d) vinylic
Answer: c)
Explanation: The circled C atom is sp3 hybridised and is attached directly to an aromatic ring, hence it is a benzylic carbon and the compound is a 1o benzylic halide.
Q.5. In which of the following cases will the compound be a tertiary (3°) halogen compound? (X=halogen atom)
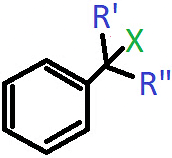
a) R’=R”=H
b) R’=CH3, R”=H
c) R’=H, R”=CH3
d) R’=R”=CH3
Answer: d)
Explanation: When R and R’ both are CH3 groups, then the carbon atom bonded to the halogen will have three alkyl groups attached to it including the benzene ring.
|
75 videos|278 docs|78 tests
|
FAQs on Classification & Nomenclature of Haloalkanes & Haloarenes - Chemistry Class 12 - NEET
| 1. What are haloalkanes and how are they classified? |  |
| 2. What are haloarenes and what distinguishes them from haloalkanes? |  |
| 3. How are haloalkanes named according to IUPAC nomenclature? |  |
| 4. What factors affect the bond polarity and reactivity of alkyl halides? |  |
| 5. What is the significance of classification and nomenclature of haloalkanes and haloarenes in organic chemistry? |  |

















