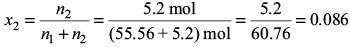Question for Competition Level Test: Solutions
Try yourself:Which one of the following aqueous solutions will exhibit highest boiling point?
Explanation
Larger the number of species in solution, larger the boiling point. 0.01 M Na2SO4 solution has 0.03 M of species
Report a problem
Question for Competition Level Test: Solutions
Try yourself:6.02 x 1020 molecules of urea are present in 100 mL of its solution. The concentration of urea solution is
(Avogadro constant, NA = 6.02 x 1023 mol–1)
Explanation
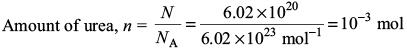
Concentration of urea solution,
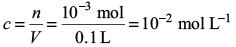
Report a problem
Question for Competition Level Test: Solutions
Try yourself:Which of the following liquid pairs shows a positive deviation from Raoult’s law?
Explanation
The molecular interactions between benzene and methanol are weaker than those existing between benzene and benzene, and methanol and methanol. Consequently, the solution will exhibit positive deviations from Raoult’s law.
Report a problem
Question for Competition Level Test: Solutions
Try yourself:Which one of the following statements is false?
Explanation
The freezing point depression is given by –ΔTf = Kf m. Different solvents have different values of Kf and hence show different freezing point depression.
Report a problem
Question for Competition Level Test: Solutions
Try yourself:If α is the degree of dissociation of Na2SO4, the van’t Hoff factor used for the calculation of molecular mass is
Explanation
Na2SO4 ionizes as
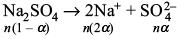
Total amount of species in solution will be n' = n(1– α) + 2nα + nα = n(1+ 2α)
The van’t Hoff factor is
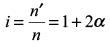
Report a problem
Question for Competition Level Test: Solutions
Try yourself:Benzene and toluene form nearly ideal solutions. At 20°C, the vapour pressure of benzene is 75 Torr and that of toluene is 22 Torr. The partial vapour pressure of benzene at 20°C for a solution containing 78 g of benzene and 46 g toluene in Torr is
Explanation
Amount of benzene,
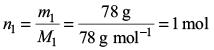
Amount of toluene,
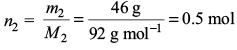
Amount fraction of benzene,
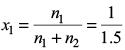
Partial pressure of benzene,
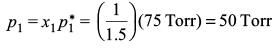
Report a problem
Question for Competition Level Test: Solutions
Try yourself:Equimolar solutions in the same solvent have
Explanation
The solutions will have the same boiling point and freezing point provided the solutes dissolved are nonelectrolytes.
Report a problem
Question for Competition Level Test: Solutions
Try yourself:Among the following mixtures, dipole-dipole as the major interaction, is present in
Explanation
Both acetonitrile and acetone are polar and have permanent dipole moment.
Report a problem
Question for Competition Level Test: Solutions
Try yourself:18 g of glucose (C6H12O6) is added to 178.2 g of water. The vapour pressure of water for this aqueous solution at 100°C is
Explanation
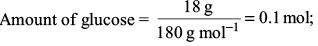
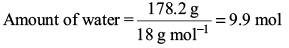
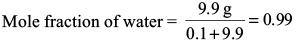
Vapour pressure of water in solution at 100°C is p = xp* = (0.99) (760 Torr) = 752.40 Torr
Report a problem
Question for Competition Level Test: Solutions
Try yourself:A 5.25% solution of a substance is isotonic with a 1.5% solution of urea (molar mass = 60 g mol–1) in the same solvent. If the densities of both the solutions are assumed to be equal to 1.0 g cm–3, molar mass of the substance will be
Explanation
Isotonic solutions have the same osmotic pressure. Hence, from
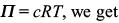
c1 = c2 ⇒ n1 = n2
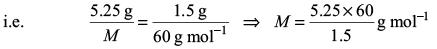
i.e. M = 210 g mol–1
Report a problem
Question for Competition Level Test: Solutions
Try yourself:In a saturated solution of the sparingly soluble electrolyte AgIO3 (relative molecular mass = 283) the equilibrium which sets in is
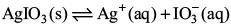
If the solubility product constant Ksp of AgIO3 at a given temperature is 1.0 x 10–8 M, what is the mass of AgIO3 contained in 100 mL of its saturated solution?
Explanation
Report a problem
Question for Competition Level Test: Solutions
Try yourself:A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mmHg at 300 K. The vapour pressure of propyl alcohol is 200 mmHg. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mmHg) at the same temperature will be
Explanation
Using the expression p = xA pA + xB pB; (A is ethyl alcohol and B is propyl alcohol)
we get 290 mmHg = 0.6 pA + 0.4 x 200 mmHg
This give
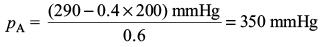
Report a problem
Question for Competition Level Test: Solutions
Try yourself:At 80 °C, the vapour pressure of pure liquid A is 520 mmHg and that of pure liquid B is 1000 mmHg. If a solution of A and B boils at 80 °C and 1 atm pressure, the amount per cent of A in the mixture is
Explanation
Let xA be the amount fraction of A in the liquid solution. We will have
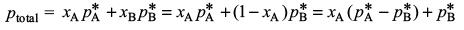
This gives
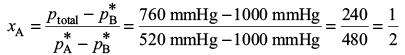
Hence, the amount per cent of A is
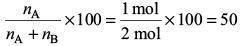
Report a problem
Question for Competition Level Test: Solutions
Try yourself:The vapour pressure of water at 20°C is 17.5 mmHg. If 18 g of glucose (C6H12O6) is added to 178.2 g of water at 20°C, the vapour of the resulting solution will be
Explanation
Amount of glucose,
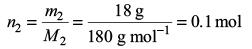
Amount of water,
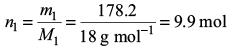
Mole fraction of water,
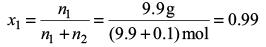
Vapour pressure of solution,
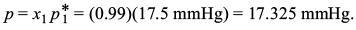
Report a problem
Question for Competition Level Test: Solutions
Try yourself:Two liquids X and Y form an ideal solution. At 300 K, vapour pressure of the solution containing 1 mol of X and 3 mol of Y is 550 mmHg. At the same temperature, if 1 mol of Y is further added to this solution, vapour pressure of the solution increases by 10 mmHg. The vapour pressures (in mmHg) of X and Y in their pure states, respectively, will be
Explanation
Report a problem
Question for Competition Level Test: Solutions
Try yourself:A binary liquid solution is prepared by mixing n-heptane and ethanol. Which one of the following statements is correct regarding the behaviour of the solution?
Explanation
The interaction between unlike molecules is weaker than those involved between like molecules. This results into positive deviations from Raoult’s law
Report a problem
Question for Competition Level Test: Solutions
Try yourself: If sodium sulphate is considered to be completely dissociated into cations and anions in aqueous solution, the decrease in freezing point of water (ΔTf) when 0.01 mol of sodium sulphate is dissolved in 1 kg of water, (Kf = 1.86 K kg mol–1) is
Explanation
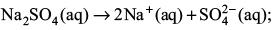
van't Hoff factor = 3
Hence,
– ΔTf = i Kf m = (3) (1.86 K kg mol–1) (0.01 mol kg–1) = 0.0558 K
Report a problem
Question for Competition Level Test: Solutions
Try yourself:On mixing, heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components (heptane and octane) are 105 kPa and 45 kPa, respectively. Vapour pressure of the solution obtained by mixing 25.0 g of heptane (molar mass = 100 g mol–1) and 35.0 g of octane (molar mass of octane = 114 g mol–1) will be
Explanation
Report a problem
Question for Competition Level Test: Solutions
Try yourself:Ethylene glycol is used as an antifreeze in a cold climate. Mass of ethylene glycol which should be added to 4 kg of water to prevent it from freezing at –6°C will be (Kf for water = 1.86 K kg mol–1 and molar mass of ethylene glycol = 62 g mol–1)
Explanation
Since
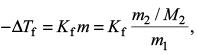
we have

Report a problem
Question for Competition Level Test: Solutions
Try yourself:The degree of dissociation (α) of a weak electrolyte AxBy is related to van’t Hoff factor (i) by the expression
Explanation
Report a problem
Question for Competition Level Test: Solutions
Try yourself:A 5.2 molal aqueous solution of methyl alcohol, CH3OH is supplied. What is the mole fraction of methyl alcohol in the solution?
Explanation
In 5.2 molal aqueous solution, we have
n2 = 5.2 mol and n1 = (1000 g/18 g mol–1) = 55.56 mol
The mole fraction of methyl alcohol in the solution is
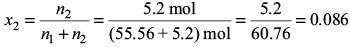
Report a problem
Question for Competition Level Test: Solutions
Try yourself:A 5 % solution of cane sugar (molar mass: 342 g mol–1) is isotonic with 1% of a solution of an unknown solute. The molar mass of unknown solute in g/mol is
Explanation
Isotonic solutions have identical osmotic pressure and hence identical concentrations, i.e. the same amounts in the fixed volume of solutions. Thus,
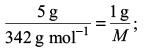
This gives
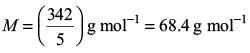
Report a problem
Question for Competition Level Test: Solutions
Try yourself:The molality of a urea solution in which 0.0100 g of urea, [(NH2)2CO], is added to 0.3000 dm3 of water at STP is
Explanation
Assuming density of water equal to 1 kg dm–3, we have

Report a problem
Question for Competition Level Test: Solutions
Try yourself:Kf for water is 1.86 K kg mol–1. If your automobile radiator holds 1.0 kg of water, how many grams of ethylene glycol (C2H6O2) must you add to get the freezing point of the solution lowered to – 2.8°C?
Explanation
Since – ΔTf = Kf m, we get.
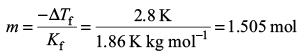
Since the molality, m = n/m1 , we get
n = mm1 = (1.505 mol kg–1) (1 kg) = 1.505 mol
Finally, the mass of ethylene glycol, C2H6O2 (the molar mass = 62 g mol–1) required will be m = n
Mm = (1.505 mol)(62 g mol–1) = 93.3 g
Report a problem
Question for Competition Level Test: Solutions
Try yourself:Consider separate solutions of 0.500 M C2H5OH(aq), 0.100 M Mg3(PO4)2 (aq), 0.250 M KBr (aq) and 0.125 M Na3PO4(aq) at 50°C. Which statement is true about these solutions, assuming all salts to be strong electrolytes?
Explanation
All the four solutions have the same amount of the species present in the solution.

Since all of them have the concentration of total species, their osmotic pressure will have the same value.
Report a problem