Compounds of Xenon and uses of Noble Gases | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Compounds of Xenon |

|
| Xenon fluorides |

|
| Xenon Oxides |

|
| Xenon Oxyfluoride |

|
Compounds of Xenon
Xenon fluorides
The three common fluorides are XeF2, XeF4, and XeF6
(1) Xenon difluoride, XeF2
It is prepared by heating a mixture of xenon and fluorine in the molecular ratio of 2 : 1 at 673 K in a sealed nickel vessel at 1 bar pressure. On cooling quickly a colourless solid XeF2 is formed.
Xe + F2 → XeF2 ( Ni Vessel, 1 bar)
(2 : 1 ratio)
Structure

- XeF2 has linear structure.
- It involves sp3d hybridisation of xenon which adopts trigonal bipyramidal geometry in which three positions are occupied by lone pairs.
Because of the symmetrical arrangement of three lone pairs (each at an angle of 120°C), the net repulsions on the Xe—F bond pairs is zero. Thus XeF2 has linear geometry.
Reaction with water
XeF2 hydrolyses slowly but completely in acidic, neutral or alkaline solutions.
(2) Xenon tetrafluoride, XeF4
It is prepared by heating a mixture of xenon and fluorine, in the molar ratio of 1 : 5, in nickel vessel, at 873 K under a pressure of 6-7 bar pressure.
Xe + 2F2 → XeF4
Structure

- XeF4 has square planar structure.
- It involves sp3d2 hybridisation of xenon which adopts octahedral geometry in which two positions are occupied by lone pairs.
Reaction with water
XeF4 undergoes slow hydrolysis with moisture and forms XeO3 which is highly explosive.
But, if the reaction is controlled at – 80° C, it forms xenon oxyfluoride;
(3) Xenon hexafluoride, XeF6
It is prepared by heating xenon with excess of fluorine (in the molar ratio of 20) in a nickel vessel at 573 K under pressure of 60-70 bar pressure.
Xe + 3F2 → XeF6
(1 : 20 ratio)
Structure
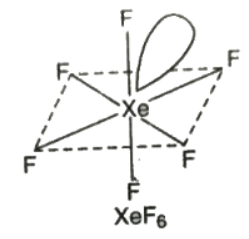
- XeF6 has distorted octahedral structure in which all the six positions are occupied by fluorine atoms and the lone pair is present at the centre of one of the triangular faces.
- It involves sp3d2 hybridisation of xenon.
Reaction with water
It undergoes slow hydrolysis with atmospheric moisture and forms XeO, which is highly explosive.
Careful hydrolysis in nickel vessel gives colourless stable liquid, XeOF4
Xenon Oxides
The important oxides of xenon are XeO3 and XeO4.
(1) Xenon trioxide, XeO3
Xenon trioxide is prepared by the hydrolysis of XeF6 or XeF4.
Structure

- It has pyramidal structure.
- It involves sp3 hybridisation of xenon.
Reaction with water
- It is soluble in water and quite stable in aqueous solution. However, it explodes violently when dry.
- XeO3 reacts with aqueous alkali to form the hydrogen xenate ion HXeO4¯, which slowly disproportionates to give xenon and perxenate ion, XeO64– in which xenon has oxidation state +8.

Perxenate solutions are yellow and are powerful oxidizing agents.
(2) Xenon tetraoxide, XeO4
It is prepared by the action of conc. H2SO4 on sodium or barium xenate (Na4XeO6 or Ba2XeO6) at room temperature.
Structure
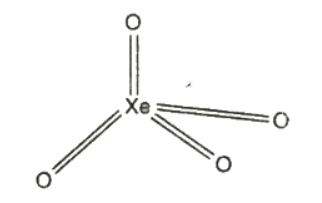
XeO4 molecule has tetrahedral structure.
Xenon Oxyfluoride
The common oxyfluorides of xenon are: XeOF2, XeOF4, and XeO2F2.
(1) Xenon oxydifluoride, XeOF2
It is prepared by the slow and partial hydrolysis of XeF4 at low temperature.
Structure
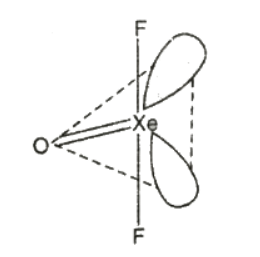
- It has T-shaped structure.
- Xenon involves sp3d hybridisation giving trigonal bipyramidal geometry in which two positions are occupied by lone pairs.
(2) Xenon oxytetrafluoride, XeOF4
- It is prepared by the partial hydrolysis of xenon hexafluoride:

- It can also be prepared by the action of XeF6 on silicon dioxide.

The contents are immediately quenched with solid CO2, as soon as the yellow colour of XeF6 disappears. It is done to avoid the formation of XeO3 (explosive) as:
Structure

- It has square pyramidal.
- It involves sp3d2 hybridisation of xenon and one position is occupied by a lone pair.
Reaction with water
It reacts with water giving xenon dioxyfluoride XeO2F2 which further reacts with water to give explosive compound XeO3 as:
FAQs on Compounds of Xenon and uses of Noble Gases - Chemistry Optional Notes for UPSC
| 1. What are xenon fluorides and what are their uses? |  |
| 2. How are xenon oxides formed and what are their properties? |  |
| 3. What is xenon oxyfluoride and what are its applications? |  |
| 4. How are xenon fluorides and xenon oxides used in the field of superconductivity? |  |
| 5. Can xenon fluorides and xenon oxides be used in industrial applications? |  |

|
Explore Courses for UPSC exam
|

|



















