Conductance of Electrolytic Solutions & Measurement of Conductivity of Ionic Solutions | Physical Chemistry for NEET PDF Download
| Table of contents |

|
| Conductance |

|
| Measurement of the Conductivity of Ionic Solutions |

|
| MOLAR IONIC CONDUCTIVITY |

|
| QUESTION ON MOLAR CONDUCTIVITY |

|
| UNITS OF MOLAR CONDUCTIVITY |

|
| DEFINATION OF MOLAR CONDUCTIVITY |

|
Conductance
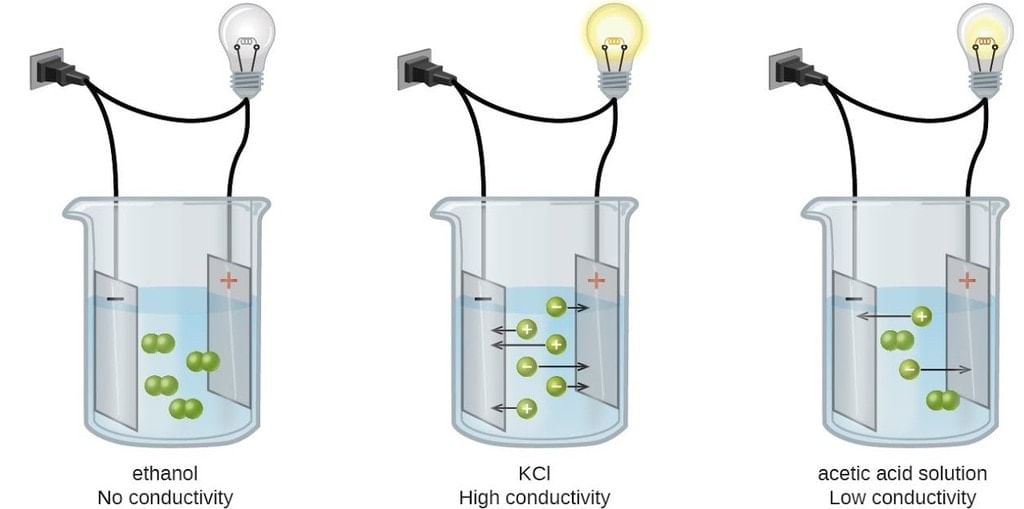 Conductance/ Conductivity of Different Electrolytic Solutions
Conductance/ Conductivity of Different Electrolytic Solutions
Introduction:
Both metallic and electrolytic conductors obey Ohm's law
i.e. V = IR
where V = Potential difference in volt; I Current in ampere ; R = resistance in Ohm
We know, resistance is directly proportional to length of conductor and inversely proportional to cross sectional area of the conductor.

Specific resistance is the resistance of a conductor having lengths of 1 cm and cross sectional area of 1 cm2.
Unit of R is ohm and unit of specific resistance is ohm cm
Reciprocal of resistance is called as conductance and reciprocal of specific resistance is called as specific conductance.

where C = conductance ohm-1; K = specific conductance ohm-1 cm-1.
Ohm and siemens are other units of conductance.

Specific conductance = Cell constant x Conductance.
Specific Conductance is Conductance of 1 cm3 of an electrolyte solution.
In case of electrolytic solution, the specific conductance is defined as the conductance of a solution of definite concentration enclosed in a cell having two electrodes of unit area separated by 1 cm apart.
1. Equivalent Conductance
Equivalent conductance is the conductance of an electrolyte solution containing 1 gm equivalent of electrolyte. It is denoted by ^.
^ = K × V
( ^ = ohm-1 cm-1 × cm3 = ohm-1 cm2)
Usually concern ration of electrolyte solution is expresses as C gm equivalent per litre.
Thus, V = 
{Volume having 1 gm equivalent electrolyte in the solution} Thus, ^ = K ×  .
.
2. Molar Conductance
Molar conductance may be defined as conductance of an electrolyte solution having 1 gm mole electrolyte in a litre. It is denoted by ^m.
^m = K × V
Usually concentration of electrolyte solution is expressed as 'M' gm mole electrolyte per litre.
Thus, V = 
Hence, ^m = K × 
Relation between ^ and ^m : ^m = n × ^
Determination of ^m0 or ^0
A plot of ^m vs as found experimentally is as shown below graphically.

the ^m vs  plot of strong electrolyte being linear it can be extrapolated to zero concentration.
plot of strong electrolyte being linear it can be extrapolated to zero concentration.
Thus, ^m values of the solution of the test electrolyte are determined at various concentrations the concentrations should be as low as good.
^m values are then plotted against  when a straight line is obtained. This is the extrapolated to zero concentration. The point where the straight line intersects ^m axis is ^ºm of the strong electrolyte.
when a straight line is obtained. This is the extrapolated to zero concentration. The point where the straight line intersects ^m axis is ^ºm of the strong electrolyte.
However, the plot in the case weak electrolyte being non linear, shooting up suddenly at some low concentration and assuming the shape of a straight line parallel to ^m axis. Hence extrapolation in this case is not possible. Thus, ^0 of a weak electrolyte cannot be determined experimentally. It can, however, be done with the help of Kohlrausch's law to be discussed later.
Measurement of the Conductivity of Ionic Solutions
MOLAR IONIC CONDUCTIVITY
QUESTION ON MOLAR CONDUCTIVITY
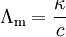
UNITS OF MOLAR CONDUCTIVITY
DEFINATION OF MOLAR CONDUCTIVITY
|
117 videos|225 docs|239 tests
|
FAQs on Conductance of Electrolytic Solutions & Measurement of Conductivity of Ionic Solutions - Physical Chemistry for NEET
| 1. What is conductance? |  |
| 2. How is the conductivity of ionic solutions measured? |  |
| 3. What are electrolytic solutions? |  |
| 4. Why is measuring the conductivity of ionic solutions important? |  |
| 5. How does temperature affect the conductivity of ionic solutions? |  |

|
Explore Courses for NEET exam
|

|


















